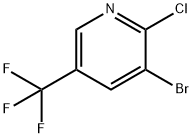
3-Bromo-2-chloro-5-(trifluoromethyl)pyridine synthesis
- Product Name:3-Bromo-2-chloro-5-(trifluoromethyl)pyridine
- CAS Number:71701-92-3
- Molecular formula:C6H2BrClF3N
- Molecular Weight:260.44
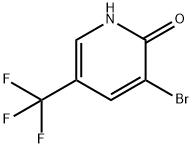
76041-73-1
143 suppliers
$9.00/1g

71701-92-3
160 suppliers
$5.00/1g
Yield: 79%
Reaction Conditions:
Stage #1:3-bromo-5-(trifluoromethyl)pyridin-2-ol with trichlorophosphate at 100; for 5 h;
Stage #2: with sodium hydrogencarbonate in dichloromethane;water
Steps:
B.2
A mixture of 3-bromo-5-(trifluoromethyl)pyridin-2-ol (37.75g, 0.16 mol) and phosphorus(lll) oxychloride (POCI3; 75 mL) is stirred at 1000C for 5 hours. After cooling to room temperature, the mixture is poured into ice-water, and extracted with CH2CI2 twice. The combined organic layer is washed with NaHCO3 aq., brine, dried over MgSO4, filtered and concentrated in vacuo. The crude mixture is purified by flash column chromatography to give 3-bromo-2-chloro-5-trifluoromethylpyridine (31.90 g, 79 % yield) as a white solid. 1H-NMR (400MHz, CDCI3), δ (ppm): 8.17 (m, 1H), 8.62 (d, 1 H).
References:
Location in patent:Page/Page column 75
79456-30-7
134 suppliers
$6.00/250mg

71701-92-3
160 suppliers
$5.00/1g

76041-73-1
143 suppliers
$9.00/1g

71701-92-3
160 suppliers
$5.00/1g