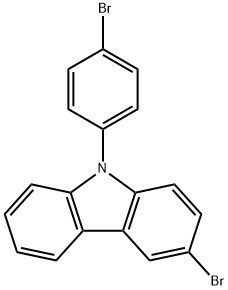
3-BroMo-9-(4-broMophenyl)carbazole synthesis
- Product Name:3-BroMo-9-(4-broMophenyl)carbazole
- CAS Number:1226860-66-7
- Molecular formula:C18H11Br2N
- Molecular Weight:401.09
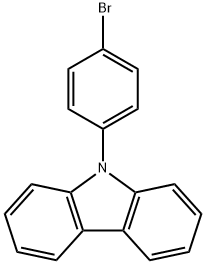
57102-42-8

1226860-66-7
General procedure for the synthesis of 3-bromo-9-(4-bromophenyl)-9H-carbazole from 9-(4-bromophenyl)carbazole: 9-(4-bromophenyl)-9H-carbazole (3.00 g, 9.31 mmol) was dissolved in dichloromethane (50 mL) under the condition of light avoidance, followed by addition of N-bromosuccinimide (NBS, 1.80 g, 10 mmol). The reaction mixture was stirred at room temperature for 12 hours. Upon completion of the reaction, water was added to the mixture and a white precipitate was precipitated. After filtration and drying, the resulting white solid was purified by recrystallization from petroleum ether. Yield: 100%.
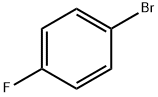
1592-95-6
403 suppliers
$5.00/1g
460-00-4
636 suppliers
$6.00/10g

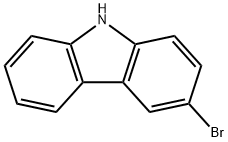
1226860-66-7
61 suppliers
$32.00/100mg
Yield: 73%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 150; for 24 h;
Steps:
Metal-Free N-Arylation of Carbazoles; General Procedure
General procedure: A mixture of a fluorinated aryl halide (2.0 mmol), a carbazole (0.5 mmol), and a base (2.0 mmol) in solvent (2 mL) was allowed to react under air atmosphere. The reaction mixture was heated to the specified temperature for 24 h. After reaction completion, the mixture was added to brine (15 mL) and extracted with CH2Cl2 (3 × 15 mL). The combined extract was concentrated under reduced pressure and the product was isolated by short chromatography on a silica gel (200-300 mesh) column.
References:
Wang, Lei;Ji, Enhui;Liu, Ning;Dai, Bin [Synthesis,2016,vol. 48,# 5,p. 737 - 750]

57102-42-8
278 suppliers
$5.00/250mg

1226860-66-7
61 suppliers
$32.00/100mg

1592-95-6
403 suppliers
$5.00/1g
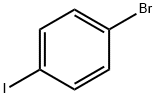
589-87-7
540 suppliers
$10.00/1g

1226860-66-7
61 suppliers
$32.00/100mg

589-87-7
540 suppliers
$10.00/1g

1226860-66-7
61 suppliers
$32.00/100mg
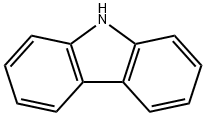
86-74-8
347 suppliers
$10.00/5g

1226860-66-7
61 suppliers
$32.00/100mg