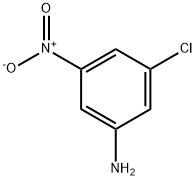
3-chloro-5-nitro-aniline synthesis
- Product Name:3-chloro-5-nitro-aniline
- CAS Number:5344-44-5
- Molecular formula:C6H5ClN2O2
- Molecular Weight:172.57
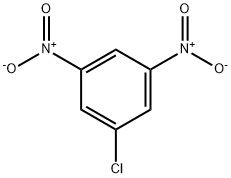
618-86-0

5344-44-5
General procedure for the synthesis of 3-chloro-5-nitroaniline from 1-chloro-3,5-dinitrobenzene: 1-chloro-3,5-dinitrobenzene (0.50 g, 2.47 mmol) was dissolved in a mixed solvent of 6 mL of ethanol and 3 mL of 20% aqueous ammonium sulphide, and the reaction was carried out at reflux for 1 hour. After completion of the reaction, diluted by adding appropriate amount of water, filtered to collect the solid product and dried. The product was purified by rapid chromatography on silica gel column using 10% acetone-petroleum ether mixed solvent as eluent, and finally 3-chloro-5-nitroaniline (0.36 g, 84% yield) was obtained as an orange powder.

618-86-0
25 suppliers
inquiry

5344-44-5
51 suppliers
inquiry
Yield: 84%
Reaction Conditions:
with diammonium sulfide in ethanol;water for 1 h;Heating / reflux;
Steps:
1 Example 1
Synthesis of 5-(R)-(4-bromobenzyl)-3-(3-chloro-5-nitrophenyl)-5-methylimidazoline-2,4-dione
The above product (0.50 g, 2.47 mmol) was refluxed 1 hr in 6 mL alcohol and 3 mL 20% ammonium sulfide in H2O. Water was added and the product was filtered, dried and flash chromatographed on silica gel eluting with 10% acetone-pet ether to obtain 3-chloro-5-nitroaniline (0.36 g, 84%) as an orange powder.
References:
Boehringer Ingelheim Pharmaceuticals, Inc. US6353013, 2002, B1 Location in patent:Page column 28
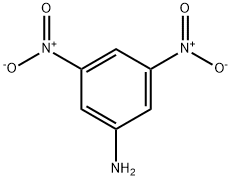
618-87-1
11 suppliers
$90.00/1g

5344-44-5
51 suppliers
inquiry
99-65-0
16 suppliers
$31.39/442244

5344-44-5
51 suppliers
inquiry

64-17-5
825 suppliers
$10.00/50g

618-86-0
25 suppliers
inquiry
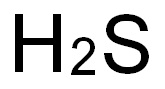
7783-06-4
77 suppliers
$133.00/25ml

7664-41-7
523 suppliers
$15.00/100ml

5344-44-5
51 suppliers
inquiry