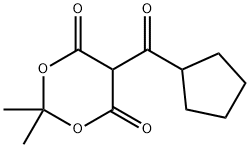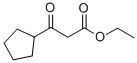
3-CYCLOPENTYL-3-OXO-PROPIONIC ACID ETHYL ESTER synthesis
- Product Name:3-CYCLOPENTYL-3-OXO-PROPIONIC ACID ETHYL ESTER
- CAS Number:24922-00-7
- Molecular formula:C10H16O3
- Molecular Weight:184.23
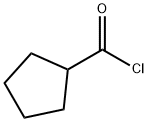
4524-93-0

24922-00-7
6a) A hexane solution of 2.4 M n-butyllithium (92.0 mL, 230 mmol) was slowly added dropwise to a solution containing monoethyl malonate (13.6 mL, 115 mmol) and a catalytic amount of 2,2'-bipyridine at -55 to -65 °C. After the dropwise addition, cyclopentanecarbonyl chloride (7.0 mL, 58 mmol) was added in batches. The reaction mixture was gradually warmed to room temperature with stirring and subsequently poured into a mixture of 1N aqueous hydrochloric acid solution and ether. The organic layer was separated, washed three times with saturated sodium bicarbonate solution, dried over anhydrous magnesium sulfate, concentrated and then purified by silica gel column chromatography (eluent: 0-5% ethyl acetate in hexane solution with gradient elution) to afford ethyl 3-cyclopentyl-3-oxopropanoate (9.50 g, 89% yield).1H-NMR (400 MHz, DMSO-d6) δ 4.06 (q, J = 7 Hz. 2H), 3.60 (s, 2H), 2.99-2.91 (m, 1H), 1.78-1.47 (m, 8H), 1.15 (t, J = 7 Hz, 3H).

4524-93-0
146 suppliers
$13.43/1gm:

24922-00-7
91 suppliers
inquiry
Yield:24922-00-7 89%
Reaction Conditions:
Stage #1:hydrogen ethyl malonate with [2,2]bipyridinyl;n-butyllithium in hexanes at -65 - -55;
Stage #2:Cyclopentanecarboxylic acid chloride in hexanes at 20;
Steps:
6.6a
6a) Ethyl 3-cyclopentyl-3-oxopropanoate To a solution of monoethyl malonate (13.6 mL, 115 mmol) and a few milligrams of 2,2'-bipyridyl at between -55 and -65° C. was slowly added a 2.4 M solution of n-butyl lithium in hexanes (92.0 mL, 230 mmol). After the addition was complete, cyclopentanecarbonyl chloride (7.0 mL, 58 mmol) was added in portions. The solution was then allowed to stir while warming to ambient temperature and was poured into a mix of 1 N aqueous hydrochloric acid and ether. The layers were separated and the ether layer was washed three times with saturated sodium bicarbonate, dried over magnesium sulfate, concentrated and purified by chromatography (silica gel, 0-5% ethyl acetate in hexanes gradient elution) to afford ethyl 3-cyclopentyl-3-oxopropanoate (9.50 g, 89%). 1H-NMR (400 MHz, DMSO-d6) δ 4.06 (q, J=7 Hz, 2H), 3.60 (s, 2H), 2.99-2.91 (m, 1H), 1.78-1.47 (m, 8H), 1.15 (t, J=7 Hz, 3H).
References:
SmithKline Beecham Corporation US2008/96921, 2008, A1 Location in patent:Page/Page column 42

4524-93-0
146 suppliers
$13.43/1gm:
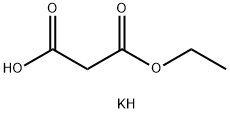
6148-64-7
400 suppliers
$5.00/10g

24922-00-7
91 suppliers
inquiry

4524-93-0
146 suppliers
$13.43/1gm:

141-78-6
683 suppliers
$20.50/250ml

24922-00-7
91 suppliers
inquiry

105-53-3
791 suppliers
$5.00/25g

24922-00-7
91 suppliers
inquiry