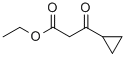
3-CYCLOPROPYL-3-OXO-PROPIONIC ACID ETHYL ESTER synthesis
- Product Name:3-CYCLOPROPYL-3-OXO-PROPIONIC ACID ETHYL ESTER
- CAS Number:24922-02-9
- Molecular formula:C8H12O3
- Molecular Weight:156.18
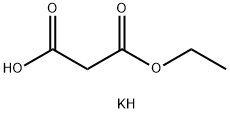
6148-64-7
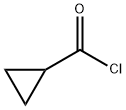
4023-34-1

24922-02-9
Under cooling conditions in an ice bath, monoethyl malonate potassium salt (17.0 g, 0.10 mol) was suspended in ethyl acetate (100 ml) and triethylamine (34.7 ml, 0.25 mol) and magnesium chloride (14.3 g, 0.15 mol) were added sequentially. The resulting mixture was stirred and reacted at 40°C for 20 hours. Cyclopropanecarbonyl chloride (4.30 g, 50.0 mmol) was also taken and dissolved in tetrahydrofuran (50 ml), oxalyl chloride (4.36 ml, 50.0 mmol) and a catalytic amount of N,N-dimethylformamide were added under the cooling of an ice bath, stirred for 1 h. After stirring, it was moved to room temperature and the reaction was continued for 1 h. The reaction was continued at room temperature. Subsequently, the previously prepared malonic acid solution was slowly added to this chloride solution under ice bath cooling and the reaction was stirred at room temperature for 20 hours. After completion of the reaction, the mixture was poured into 10% aqueous citric acid solution (300 ml) and extracted with ethyl acetate (300 ml x 3). The organic layers were combined and washed sequentially with saturated aqueous sodium bicarbonate (500 ml) and brine (300 ml) and dried over anhydrous sodium sulfate. The solvent was concentrated under reduced pressure to give ethyl 3-cyclopropyl-3-carbonyl-propionate 7.26 g (93% yield) as a colorless oil, which was used directly in the next reaction.1H-NMR (400 MHz, CDCl3) δ: 0.94-0.99 (2H, m), 1.10-1.15 (2H, m), 1.28 (3H, t, J=7.08 Hz). 2.01-2.06 (1H, m), 3.57 (2H, s), 4.21 (2H, q, J=7.08Hz).

6148-64-7
408 suppliers
$5.00/10g

4023-34-1
373 suppliers
$12.00/5g

24922-02-9
209 suppliers
$5.00/1g
Yield:24922-02-9 93%
Reaction Conditions:
Stage #1:ethyl potassium malonate with triethylamine;magnesium chloride in ethyl acetate at 0 - 40; for 20 h;
Stage #2:cyclopropanecarboxylic acid chloride in tetrahydrofuran;ethyl acetate at 0 - 20; for 20 h;
Steps:
73 Reference Example 73; Ethyl 3-cyclopropyl-3-oxopropionate (I-73)
To ethyl acetate (100 ml) suspension of potassium ethyl malonate (17.0 g, 0.10 mol) were added triethylamine (34.7 ml, 0.25 mol) and magnesium chloride (14.3 g, 0.15 mol) with cooling with ice, and then the resulting mixture was stirred at 40°C for 20 hours. To tetrahydrofuran (50 ml) solution of cyclopropanecarboxylicacid (4.30 g, 50.0 mmol) were added oxalyl chloride (4.36 ml, 50.0 mmol) and a catalytic amount of N,N-dimethylformamide with cooling with ice, and the resulting mixture was stirred as such for 1 hour and then at room temperature for 1 hour. The above-mentioned malonic acid solution was added to this acid chloride solution with cooling with ice, and the resulting mixture was stirred at room temperature for 20 hours. The reaction mixture was poured into 300 ml of aqueous 10 % citric acid solution, and the mixture was extracted with ethyl acetate (300 ml × 3). The organic layer was successively washed with 500 ml of aqueous saturated sodium bicarbonate solution and 300 ml of brine, and dried over sodium sulfate. The solvent was evaporated, and7.26g (93%) of the entitled compound was obtained as a colorless oil (this was directly used in the next reaction).1H-NMR(400MHz, CDCl3)δ: 0.94-0.99(2H, m), 1.10-1.15(2H, m), 1.28(3H, t, J=7.08Hz), 2.01-2.06(1H, m), 3.57 (2H, s), 4.21(2H, q, J=7.08Hz).
References:
DAIICHI PHARMACEUTICAL CO., LTD. EP1479681, 2004, A1 Location in patent:Page 59
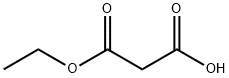
1071-46-1
288 suppliers
$5.00/1g

4023-34-1
373 suppliers
$12.00/5g

24922-02-9
209 suppliers
$5.00/1g
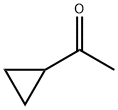
765-43-5
411 suppliers
$10.00/1g
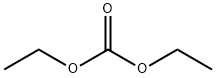
105-58-8
480 suppliers
$14.00/25g

24922-02-9
209 suppliers
$5.00/1g