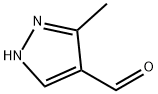
3-METHYL-1H-PYRAZOLE-4-CARBALDEHYDE synthesis
- Product Name:3-METHYL-1H-PYRAZOLE-4-CARBALDEHYDE
- CAS Number:112758-40-4
- Molecular formula:C5H6N2O
- Molecular Weight:110.11

68-12-2
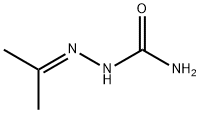
110-20-3

112758-40-4
N,N-dimethylformamide (65 mL) was cooled at 0 °C and trichlorophosphorus (39 mL) was slowly added dropwise over a period of 30 minutes and stirring was continued at 0 °C for 1 hour. Subsequently, 2-(propane-2-ylidene)hydrazinecarboxamide (13 g, 114 mmol) was added in batches at 0 °C, and then the reaction mixture was warmed up to 70 °C and kept for 4 hours. Upon completion of the reaction, the mixture was poured into crushed ice (500 g) and neutralized to neutral with 10% sodium hydroxide solution, followed by extraction with ethyl acetate (3 x 150 mL). The organic phases were combined, washed sequentially with water (2 x 100 mL) and saturated aqueous sodium chloride solution (100 mL), dried over anhydrous sodium sulfate and concentrated to give the crude product. The crude product was purified by column chromatography (60-120 mesh silica gel), using chloroform solution containing 2-4% methanol as eluent, and finally 3-methyl-1H-pyrazole-4-carboxaldehyde (4 g, 32% yield) was obtained as solid. The product was characterized by 1H NMR (CDCl3): δ 9.96 (s, 1H), 8.0 (s, 1H), 2.6 (s, 3H).
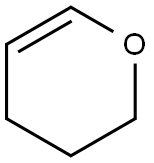
110-87-2
544 suppliers
$10.00/1g
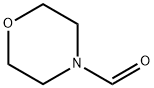
4394-85-8
327 suppliers
$6.00/25g
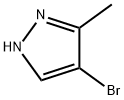
13808-64-5
203 suppliers
$6.00/1g

112758-40-4
156 suppliers
$19.00/250mg
Yield: 8 g
Reaction Conditions:
Stage #1:3,4-dihydro-2H-pyran;4-bromo-3-methyl-1H-pyrazole with trifluoroacetic acid for 12 h;Reflux;
Stage #2: in tetrahydrofuran at -75; for 0.5 h;
Stage #3:4-morpholinecarboxaldehydeFurther stages;
Steps:
2.2
(1) Combine 16.1 g (0.1 mol) of 4-bromo-3-methyl-1H-pyrazole, 10.1 g (0.12 mol) of 3,4-dihydro-2H-pyran, and 0.7 g (0.006 mol) of trifluoroacetic acid ) Add to the reaction bottle and stir to heat to micro-reflux for 12 hours. The reaction formula is as follows:Cool to room temperature, add 0.24 g (0.006 mol) of sodium hydride to neutralize the reaction system,Vacuum distillation to obtain intermediate4-bromo-3-methyl-1- (tetrahydro-2H-pyran-2-yl) -1H-pyrazole and4-bromo-5-methyl-1- (tetrahydro-2H-pyran-2-yl) -1H-pyrazole total 24 grams,The presence of isomers does not affect the next reaction. (2) The intermediates obtained in step (1) 4-bromo-3-methyl-1- (tetrahydro-2H-pyran-2-yl) -1H-pyrazole and 4-bromo-5- Methyl-1- (tetrahydro-2H-pyran-2-yl) -1H-pyrazole 24 g was dissolved in 200 ml of THF, cooled to minus 75 degrees, and n-butyl with a concentration of 2.5 mol / L was added dropwise 48 ml (0.12 mol) of lithium solution, maintaining the temperature at minus 75 degrees Celsius, dropping and holding for half an hour, then adding 14 g (0.12 mol) of N-formylmorpholine at this temperature, the reaction formula in step (2) is as follows :After the reaction is completed, naturally warm to room temperature, add dilute sulfuric acid to quench until the system pH value is less than 1, stir at room temperature for 3-4 hours, neutralize with sodium bicarbonate, extract with ethyl acetate, dry and concentrate to obtain the target product by adsorption and purification on silica gel 3-Methyl-1H-pyrazole-4-carbaldehyde 8 g.The yield of the target product 3-methyl-1H-pyrazole-4-carbaldehyde in this example was 72.7%. 2 is the nuclear magnetic resonance spectrum of the product 3-methyl-1H-pyrazole-4-carbaldehyde obtained in this example;
References:
Changsha Luxing Biological Technology Co., Ltd.;Tan Yongjun;Yang Bing;Zhu Zhiping;Zhang Jianhua CN110734401, 2020, A Location in patent:Paragraph 0030-0039
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

112758-40-4
156 suppliers
$19.00/250mg

112758-40-4
156 suppliers
$19.00/250mg
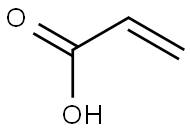
79-10-7
737 suppliers
$20.16/100 mL