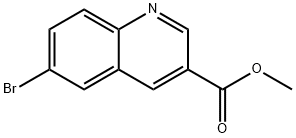
3-Quinolinecarboxylic acid, 6-broMo-, Methyl ester synthesis
- Product Name:3-Quinolinecarboxylic acid, 6-broMo-, Methyl ester
- CAS Number:1220418-77-8
- Molecular formula:C11H8BrNO2
- Molecular Weight:266.09

67-56-1
798545-30-9

1220418-77-8
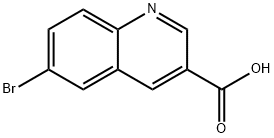
Example 1: Synthesis of tert-butyl {3-[(hydroxyamino)carbonyl]quinolin-6-yl}carbamate (Compound 1-21, Int-2) Step 1: Synthesis of methyl 6-bromoquinoline-3-carboxylate (Intermediate 1) [00161] To a solution of 6-bromoquinoline-3-carboxylic acid (3 g, 11.9 mmol) in methanol (55 mL) was slowly added concentrated sulfuric acid (0.32 mL, 6 mmol). The reaction mixture was stirred and refluxed at 85 °C for 96 hours. Upon completion of the reaction, the mixture was cooled to room temperature and the pH was adjusted to 7 by slow addition of saturated sodium bicarbonate solution.During neutralization, methyl 6-bromoquinoline-3-carboxylate precipitated as a solid, which was collected by filtration (Intermediate 1, 2.77 g, 97% yield). Product Characterization: LC-MS (FA) ES+ m/z: 266 [M+H]+. 1H NMR (400 MHz, DMSO-d6) δ ppm: 9.33 (d, J = 2.1 Hz, 1H), 9.01 (d, J = 2.1 Hz, 1H), 8.53 (t, J = 1.3 Hz, 1H), 8.07-8.01 (m, 2H), 3.95 (s, 3H).

67-56-1
790 suppliers
$7.29/5ml-f

798545-30-9
73 suppliers
$60.00/100 mg

1220418-77-8
42 suppliers
$50.00/25mg
Yield: 97%
Reaction Conditions:
Stage #1:methanol;6-bromoquinoline-3-carboxylic acid with sulfuric acid at 85; for 96 h;
Stage #2: with sodium hydrogencarbonate; pH=7 at 20;
Steps:
5.1.1
Example 1: tert-but l {3-[(hydroxyamino)carbonyI]quinolin-6-yl}carbamate Compound 1-21 lnt-2 Step 1: methyl 6-bromoquinoline-3-carboxylate Intermediate 1 [00161] To a solution of 6-bromoquinoline-3-carboxylic acid (3 g, 1 1.9 mmol), in methanol (55 mL) was added sulfuric acid (0.32 mL, 6 mmol). The reaction was stirred at 85 °C for 96 h. The reaction was then cooled to room temperature and saturated sodium bicarbonate solution was added until pH = 7. On neutralization, methyl 7-bromo-2-naphthoate precipitated as a solid which was collected by suction filtration (Int-l , 2.77 g, 97 %). LC-MS: (FA) ES+ 266; .H NMR (400 MHz, i 6-DMSO) δ ppm 9.33 (d, J = 2.1 Hz, 1 H), 9.01 (d, J = 2.1 Hz, 1 H), 8.53 (t, J = 1.3, 1.3 Hz, 1 H), 8.07-8.01 (m, 2 H), 3.95 (s, 3 H).
References:
MILLENNIUM PHARMACEUTICALS, INC.;GOULD, Alexandra, E.;HARRISON, Sean, J. WO2011/146591, 2011, A1 Location in patent:Page/Page column 60
1001-26-9
207 suppliers
$6.00/1g
29124-57-0
187 suppliers
$16.00/250mg

1220418-77-8
42 suppliers
$50.00/25mg

67-56-1
790 suppliers
$7.29/5ml-f

1001-26-9
207 suppliers
$6.00/1g

29124-57-0
187 suppliers
$16.00/250mg

1220418-77-8
42 suppliers
$50.00/25mg

50-00-0
896 suppliers
$10.00/25g
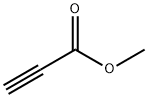
922-67-8
354 suppliers
$6.00/1g

106-40-1
483 suppliers
$12.00/5g

1220418-77-8
42 suppliers
$50.00/25mg
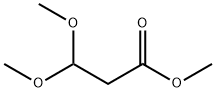
7424-91-1
263 suppliers
$9.00/1g

29124-57-0
187 suppliers
$16.00/250mg

1220418-77-8
42 suppliers
$50.00/25mg