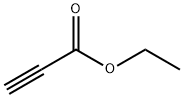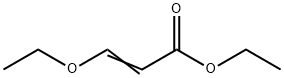
Ethyl 3-ethoxyacrylate synthesis
- Product Name:Ethyl 3-ethoxyacrylate
- CAS Number:1001-26-9
- Molecular formula:C7H12O3
- Molecular Weight:144.17
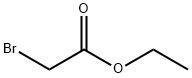
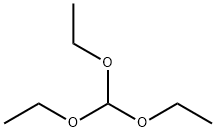
Ethyl 3-ethoxyacrylate is an intermediate in organic synthesis and pharmaceutical intermediate, which can be used in laboratory research and development process. Its can be synthesised by the substitution reaction of Ethyl bromoacetate and Triethyl orthoformate.
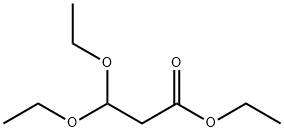
10601-80-6
315 suppliers
$6.00/5g

1001-26-9
208 suppliers
$6.00/1g
Yield:1001-26-9 100%
Reaction Conditions:
at 190 - 200; for 2 h;
Steps:
6
40 g of powder Ζη were washed with dilute hydrochloric acid once, H20 washed 3 times, washed once in methanol, acetone and once with 20 Mm Hg and 100 degrees Celsius for 10 minutes after drying, remove the nitrogen 25 g was added to 100 ml three Ζη Round-bottomed flask containing a catalytic amount of iodine was added 6 ml of anhydrous benzene was heated and stirred at reflux, was added over 45 minutes containing 8.35 grams, 0.05 mole of ethyl bromoacetate and 8.89 g XI, 0 06 mol triethylorthoformate XII. After the dropwise addition, the treated again take Ζη 6.25 g flour added to the system, after the reaction was refluxed for 6 hours, the reaction solution was transferred to a 100 ml ice-water and 50 g, excess acetic acid was added, the ether layer was separated, with washed with saturated sodium bicarbonate solution, dried over anhydrous sodium sulfate, and concentrated under 1.5 mm Hg vacuum distillation, 79-81 ° C was collected as a colorless oil distillate 3, 3-ethoxy-propionate esters XIII2 8 g, yield 29.4%; collected above to give the 2.8 g 3, 3-ethoxypropionate XIII at 190-200 degrees Celsius was heated under reflux for 2 hours with a delicate smell ., colorless liquid of ethyl acrylate, 3- XIV2 2 g, yield 100%;. the 2.2 g, 152 mmol of ethyl acrylate, 3- XIV added to 7.5 ml of water and 7.5 ml two six ring oxygen mixed solvent, -10 degrees centigrade to cool large, slowly added 2.97 g after 16.72 mmol of NBS, the reaction 1 hour at room temperature, then 1.15 g, 15.2 mmol) was added thiourea after 1 hour at 80 ° C, ice-cooling, after addition of an excess of ammonia brown solid appeared, suction filtered to give a solid, after washing with water and drying give 1.4 g 2-aminothiazol-5-carboxylate XV of sitting Jie, The yield was 53.8%
References:
Jiangxi Tianren Ecology Co., Ltd.;Nankai University;Chen, Xiaoyan;Liu, Xiping;Fan, Zhijin;Liang, Xiaowen;Li, Yuedong;Mao, Wutao;Li, Juanjuan;Wang, Dun;Wang, Shuhua;Zhou, Lifeng;Ji, Xiaotian;HUA, XUEWEN CN102942565, 2016, B Location in patent:Paragraph 0038-0039; 0091-0092

64-17-5
825 suppliers
$10.00/50g
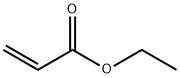
140-88-5
559 suppliers
$12.00/100ml

1001-26-9
208 suppliers
$6.00/1g

10601-80-6
315 suppliers
$6.00/5g