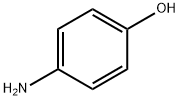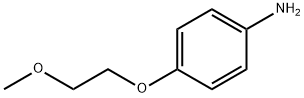
4-(2-METHOXYETHOXY)ANILINE synthesis
- Product Name:4-(2-METHOXYETHOXY)ANILINE
- CAS Number:33311-29-4
- Molecular formula:C9H13NO2
- Molecular Weight:167.21
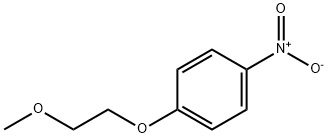
22483-40-5

33311-29-4
General procedure for the synthesis of 4-(2-methoxyethoxy)aniline from 4-nitrophenyl-2-methoxyethyl ether: 1-(2-methoxyethoxy)-4-nitrobenzene (38) (3.9 g, 20 mmol), methanol (80 mL), and 10% activated carbon (400 mg) were added to a hydrogenation reactor. The reaction mixture was stirred overnight at room temperature in a hydrogen atmosphere. Upon completion of the reaction, the mixture was filtered through diatomaceous earth to remove the activated carbon. The filtrate was concentrated in vacuum to afford 4-(2-methoxyethoxy)aniline (39) (3.1 g, 93% yield) as a black oily product. m/z (M+1) 168.1 was shown by LC-MS (ESI) analysis.

109-86-4
574 suppliers
$11.00/25g
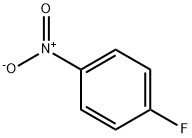
350-46-9
522 suppliers
$10.60/1gm:

33311-29-4
78 suppliers
$46.00/1 g
Yield: 76.9%
Reaction Conditions:
with dimethyl sulfoxide;potassium hydroxide at 60;
Steps:
7 Synthesis of intermediate 4- (2-methoxyethoxy) aniline
P-fluoronitrobenzene (0.5g, 3.54mmol),Ethylene glycol methyl ether (0.335ml, 4.25mmol), potassium hydroxide (0.3g, 5.31mmol) was added to 10ml of dimethyl sulfoxide, stirred at 60 overnight, water analysis, suction filtration0.537 g of a yellow solid was obtained with a yield of 76.9%.
References:
China Pharmaceutical University;Xiang Hua;Teng Yu;Li Zhenbang;Xiao Maoxu;Ren Shengnan;Ha Si;Tong Chao CN111057021, 2020, A Location in patent:Paragraph 0088-0090

22483-40-5
72 suppliers
$7.00/250mg

33311-29-4
78 suppliers
$46.00/1 g

109-86-4
574 suppliers
$11.00/25g
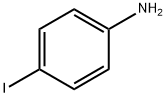
540-37-4
469 suppliers
$6.00/5g

33311-29-4
78 suppliers
$46.00/1 g

350-46-9
522 suppliers
$10.60/1gm:

33311-29-4
78 suppliers
$46.00/1 g