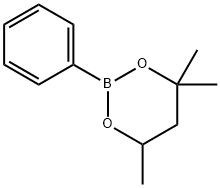
4,4,6-TRIMETHYL-2-PHENYL-1,3,2-DIOXABORINANE synthesis
- Product Name:4,4,6-TRIMETHYL-2-PHENYL-1,3,2-DIOXABORINANE
- CAS Number:15961-35-0
- Molecular formula:C12H17BO2
- Molecular Weight:204.07
![2-ISOPROPOXY-4,4,6-TRIMETHYL-[1,3,2]DIOXABORINANE](/CAS/GIF/61676-61-7.gif)
61676-61-7
48 suppliers
$37.00/5g
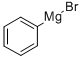
100-58-3
195 suppliers
$18.00/10ml

15961-35-0
17 suppliers
$45.00/250mg
Yield:15961-35-0 98%
Reaction Conditions:
Stage #1: 2-isopropoxy-4,4,6-trimethyl-[1,3,2] dioxaborinane;phenylmagnesium bromide in tetrahydrofuran at 20; for 2 h;Inert atmosphere;
Stage #2: with hydrogenchloride in water at 0 - 20; for 1 h;
Steps:
Synthesis of 4,4,6-trimethyl-2-phenyl-[1,3,2]dioxaborinane
Synthesis of 4,4,6-trimethyl-2-phenyl-[1,3,2]dioxaborinane To a solution of 2-isopropoxy-4,4,6-trimethyl-[1,3,2]dioxaborinane (5.58 g, 30 mmol, 1.5 eq.) in dry THF (15 ml) under an argon atmosphere a solution of phenylmagnesium bromide (1M in THF, 20 mmol) was added dropwise via addition funnel at room temperature. The reaction mixture was allowed to stir at ambient temperature for 2 hours. The reaction flask was then cooled to 0° C. and an aqueous solution of hydrochloric acid (1N, 30 ml) was added dropwise. After the addition was completed the reaction mixture was allowed to warm to ambient temperature for 1 hour. The organic layer was then separated from water layer. The latter one was extracted with ethyl acetate (3*30 ml). The combined organic layers were dried using magnesium sulfate, then filtered and concentrated under reduced pressure. The residue was purified using flash chromatography on silica gel (5% ethyl acetate:hexanes) to obtain the title compound in 98% yield. 1H NMR (400 MHz, CDCl3): δ 7.84 (d, J=7.3 Hz, 2H), 7.40 (t, J=7.3 Hz, 1H), 7.36 (t, J=7.9 Hz, 2H), 4.36 (m, 1H), 1.87 (dd, J=13.9 Hz, J=3.3 Hz, 1H), 1.62 (t, J=11.7 Hz, 1H), 1.41 (d, J=6.5 Hz, 6H), 1.38 (d, J=7.4 Hz, 3H). 11B NMR (128.3 MHz, CDCl3): δ 26.8. 13C NMR (100.6 MHz, CDCl3): δ 133.6, 130.2, 127.3, 70.8, 64.9, 45.9, 31.2, 28.1, 23.1.
References:
US2012/289733,2012,A1 Location in patent:Page/Page column 5
107-41-5
545 suppliers
$5.00/100g

98-80-6
743 suppliers
$5.00/5g

15961-35-0
17 suppliers
$45.00/250mg

108-86-1
524 suppliers
$10.00/5g
230299-21-5
149 suppliers
$9.00/1g

15961-35-0
17 suppliers
$45.00/250mg

230299-21-5
149 suppliers
$9.00/1g
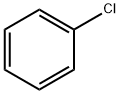
108-90-7
684 suppliers
$10.00/25G

15961-35-0
17 suppliers
$45.00/250mg

230299-21-5
149 suppliers
$9.00/1g
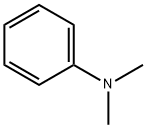
121-69-7
567 suppliers
$10.00/25mL

15961-35-0
17 suppliers
$45.00/250mg