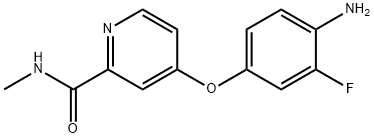
4-(4-AMINO-3-FLUOROPHENOXY)-N-METHYLPICOLINAMIDE synthesis
- Product Name:4-(4-AMINO-3-FLUOROPHENOXY)-N-METHYLPICOLINAMIDE
- CAS Number:757251-39-1
- Molecular formula:C13H12FN3O2
- Molecular Weight:261.25
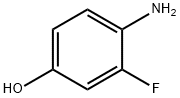
399-95-1
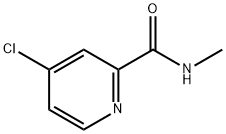
220000-87-3

757251-39-1
Under nitrogen atmosphere, 363 g of 4-amino-3-fluorophenol, 374 g of 4-chloro-N-methylpyridine-2-carboxamide and 3740 mL of N,N-dimethylacetamide were sequentially added to the reaction vessel and stirred until completely dissolved. Subsequently, 115 g of sodium hydroxide was added and the temperature of the reaction system was raised to 105 °C and the temperature was maintained for 1 hour. Upon completion of the reaction, 5600 mL of water was added to the system, the mixture was cooled to 10 °C and stirred at this temperature overnight to promote crystallization. After separation by filtration, 509 g of the brown solid product 4-(4-amino-3-fluorophenoxy)-N-methylpyridine-2-carboxamide was obtained by drying. The product was analyzed by high performance liquid chromatography (HPLC) and showed a purity of 99.3%, a melting point of 141.5-142.5 °C and a yield of 88.9%.
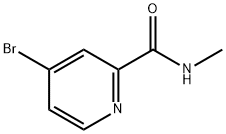
1209459-88-0
38 suppliers
$92.00/100mg

399-95-1
435 suppliers
$14.00/5g

757251-39-1
249 suppliers
$11.00/250mg
Yield:757251-39-1 97.5%
Reaction Conditions:
with N-benzyl-N,N,N-triethylammonium chloride;potassium iodide;sodium hydroxide in water at 30 - 70; for 2 h;Reagent/catalyst;
Steps:
4 Preparation of Compound III
Add 4.71g of 4-amino-3-fluorophenol, 4.13g of sodium hydroxide, 32.11g of potassium iodide, 22.78g of triethylbenzylammonium chloride, 500ml of water to the reaction flask, and heat to 30 ° C to stir and dissolve. When the temperature reaches At 70 ° C, 21.61 g of compound III was added dropwise, and the reaction was stirred for 2 h. After completion of the reaction, the mixture was cooled, washed with a 2% aqueous sodium hydroxide solution, and the mixture was separated. The organic layer was recrystallized from ethanol to give compound IV 25.48 g, product yield 97.5%, purity 99.96%.
References:
CN108329260,2018,A Location in patent:Paragraph 0014; 0025; 0028; 0031-0034
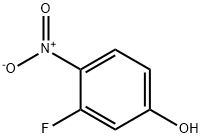
394-41-2
299 suppliers
$16.00/1g

220000-87-3
493 suppliers
$8.00/5g

757251-39-1
249 suppliers
$11.00/250mg
![Phenol, 4-[(1,2-dimethylpropylidene)amino]-3-fluoro-](/CAS/20180808/GIF/1338722-53-4.gif)
1338722-53-4
1 suppliers
inquiry

220000-87-3
493 suppliers
$8.00/5g

757251-39-1
249 suppliers
$11.00/250mg
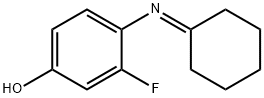
1338722-54-5
3 suppliers
inquiry

220000-87-3
493 suppliers
$8.00/5g

757251-39-1
249 suppliers
$11.00/250mg
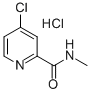
882167-77-3
125 suppliers
$9.00/1g

399-95-1
435 suppliers
$14.00/5g

757251-39-1
249 suppliers
$11.00/250mg