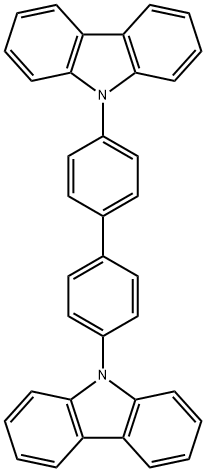
4,4'-Bis(N-carbazolyl)-1,1'-biphenyl synthesis
- Product Name:4,4'-Bis(N-carbazolyl)-1,1'-biphenyl
- CAS Number:58328-31-7
- Molecular formula:C36H24N2
- Molecular Weight:484.59
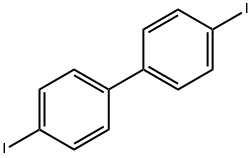
3001-15-8
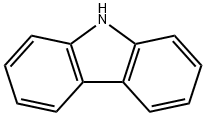
86-74-8

58328-31-7
Under nitrogen protection, 150 g (369.5 mmol) of 4,4'-diiodobiphenyl, 123.5 g (738.6 mmol) of carbazole, 23 g of copper powder, 100 μL of potassium carbonate, and 500 mL of 1,3-diisopropylbenzene were added to a 2 L triple-necked flask equipped with a stirrer and condensation device. The reaction mixture was heated to reflux and maintained for 30 hours and then naturally cooled to room temperature. Subsequently, an appropriate amount of toluene was added to the reaction mixture and the insoluble material was removed by filtration. The solvent in the filtrate was removed by distillation under reduced pressure to obtain the crude product. To the crude product, 500 mL of methanol was added for recrystallization and a solid was precipitated. The crystals were collected by filtration and dried to give 122.8 g of the target product 4,4'-bis(carbazol-9-yl)biphenyl (CBP) in 68.6% yield.
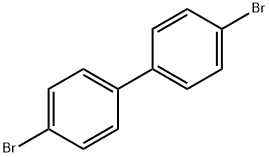
92-86-4
392 suppliers
$15.00/25g

86-74-8
347 suppliers
$10.00/5g
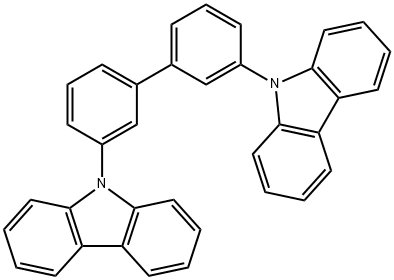
342638-54-4
131 suppliers
$45.00/2.5mg
![9H-Carbazole, 9,9'-[1,1'-biphenyl]-3,4'-diylbis-](/CAS/20210305/GIF/1325751-60-7.gif)
1325751-60-7
2 suppliers
inquiry

58328-31-7
238 suppliers
$22.00/1g
Yield:58328-31-7 77% ,342638-54-4 8.5% ,1325751-60-7 4%
Reaction Conditions:
with potassium tert-butylate in toluene at 130 - 140; for 48 h;Inert atmosphere;Autoclave;regioselective reaction;
Steps:
Synthesis of bis (9H-carbozol-9-yl)-biphenyls 2 (general procedure).
General procedure: Potassium tertbutoxide (1.12 g, 10 mmol) and carbazole (1.67 g, 10 mmol) were added to appropriate dihalobiphenyl 1 (1 mmol) in dry toluene (20 mL). The mixture was heated in a glass autoclave under argon at 130-140 C for 48 h.The resulting suspension was diluted with water to remove inorganic impurities. The organic layer was separated, concentrated to dryness, and chromatographed on silica gel using a 0-10% acetone solution in hexane.
References:
Kovalev;Pavlyuk;Zaripov;Zyryanov;Kopchuk;Rusinov;Chupakhin [Russian Chemical Bulletin,2015,vol. 64,# 8,p. 1978 - 1981][Izv. Akad. Nauk, Ser. Khim.,2015,# 8,p. 1978 - 1981,4]

3001-15-8
329 suppliers
$14.00/25g

86-74-8
347 suppliers
$10.00/5g

58328-31-7
238 suppliers
$22.00/1g
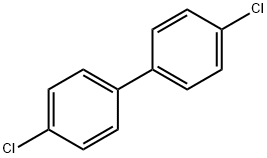
2050-68-2
82 suppliers
$23.19/u

86-74-8
347 suppliers
$10.00/5g

342638-54-4
131 suppliers
$45.00/2.5mg
![9H-Carbazole, 9,9'-[1,1'-biphenyl]-3,4'-diylbis-](/CAS/20210305/GIF/1325751-60-7.gif)
1325751-60-7
2 suppliers
inquiry

58328-31-7
238 suppliers
$22.00/1g
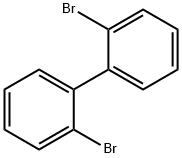
13029-09-9
285 suppliers
$20.00/1g
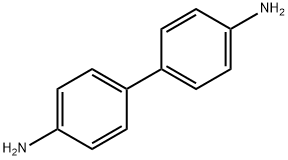
92-87-5
194 suppliers
$31.00/250mg

58328-31-7
238 suppliers
$22.00/1g