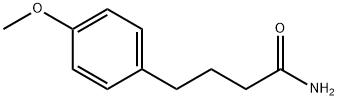
4-(4-Methoxyphenyl)butyraMide synthesis
- Product Name:4-(4-Methoxyphenyl)butyraMide
- CAS Number:2222-15-3
- Molecular formula:C11H15NO2
- Molecular Weight:193.24
Yield:2222-15-3 97%
Reaction Conditions:
with 4-methyl-morpholine;sodium chloride;ammonia in tetrahydrofuran;methanol;ethyl acetate;
Steps:
4 4-(4-Methoxyphenyl)butyramide (117)
4-(4-Methoxyphenyl)butyramide (117)
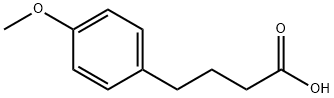
4-(4-Methoxyphenyl) butyric acid (1) (450 g, 2.32 mole) was combined with dry THF (4 L) and 4-methylmorpholine (268 mL, 2.43 mole) in a 12 L three neck flask with mechanical stirring, an ice-methanol cooling bath and nitrogen atmosphere.
Small pieces of dry ice were used to bring the bath temperature below -20° C.
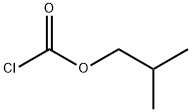
Isobutyl chloroformate was added at a rate so as not to exceed an internal temperature of -10° C.
After stirring for 30 min. at -10 to -20° C., a 4.7 M solution of ammonia in methanol (990 mL, 4.64 mole) was added in one portion.
During the addition, the reaction temperature rose to 0° C.
The reaction was allowed to stir for 30 min. and then allowed to stand overnight.
The product mixture was transferred to a 22 L separatory funnel with ethyl acetate (6 L), and 10% sodium chloride solution (1.5 L).
The layers were separated and the organic solution was washed with 10% sodium chloride solution (4*1 L) and then brine (3*500 mL).
The organic layer was dried over sodium sulfate, filtered, evaporated and placed under high vacuum overnight.
This afforded 432 g (97%) of the pure amide (117) as an off white solid.
1H NMR (300 MHz, CD3OD) δ 1.81-1.93 (m, 2H), 2.20 (t, J=7.7 Hz, 2H), 2.57 (t, J-7.7 Hz, 2H), 3.74 (s, 3H), 6.82 (d, J=8.7 Hz), 7.09 (d, J=8.7 Hz, 1H). CI MS m/z=194 [C11H15NO2+H]+.
References:
US2015/376146,2015,A1
4521-28-2
191 suppliers
$9.00/1g

2222-15-3
15 suppliers
inquiry
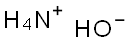
1336-21-6
693 suppliers
$14.56/250ML

4521-28-2
191 suppliers
$9.00/1g

2222-15-3
15 suppliers
inquiry
6836-18-6
26 suppliers
$600.00/5G

2222-15-3
15 suppliers
inquiry
100-66-3
506 suppliers
$10.00/5G

2222-15-3
15 suppliers
inquiry