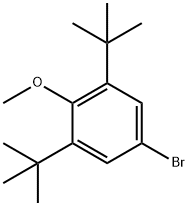
4-Bromo-2,6-di-tert-butylanisole synthesis
- Product Name:4-Bromo-2,6-di-tert-butylanisole
- CAS Number:1516-96-7
- Molecular formula:C15H23BrO
- Molecular Weight:299.25
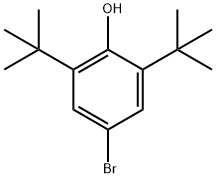
1139-52-2

77-78-1

1516-96-7
The general procedure for the synthesis of 4-bromo-2,6-di-tert-butyl anisole from 4-bromo-2,6-di-tert-butylphenol and dimethyl sulfate was as follows: 4-bromo-2,6-di-tert-butylphenol (50 g, 0.175 mol) and potassium carbonate (96.7 g, 4.0 eq.) were dissolved in acetone (750 mL) under argon atmosphere protection. Dimethyl sulfate (38.6 g, 1.75 eq.) was slowly added to the reaction system at 22 °C, followed by heating the reaction mixture to reflux and stirring for 13 hours. After completion of the reaction, the insoluble material was removed by filtration and the solvent was evaporated under reduced pressure. A partition extraction was performed by adding ethyl acetate (150 mL) and water (100 mL) to the residue. The organic layer was washed sequentially with water (100 mL), 5% aqueous NaHCO3 (100 mL) and 5% aqueous NaCl (100 mL). The organic layer was dried with anhydrous magnesium sulfate, and after filtration by gravity, the filtrate was concentrated under reduced pressure to afford 4-bromo-2,6-di-tert-butylanisole (56.1 g, brown oil) in 95.2% yield. The product was analyzed by 1H-NMR (300 MHz, CDCl3, TMS) with chemical shifts of δ 1.41 (s, 18H), 3.68 (s, 3H), 7.33 (s, 2H).
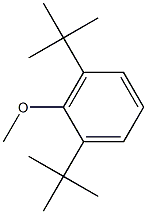
1516-95-6
14 suppliers
$41.89/10g
3848-49-5
1 suppliers
inquiry
Yield:3848-49-5 96%
Reaction Conditions:
with NBS in acetonitrile; for 20 h;
Steps:
7 5-bromo-l,3-di-tert-butyl-2-methoxybenzene
l,3-di-tert-butyl-2-methoxybenzene (Bai, Xinyan et al, Tetrahedron, 69(3), 1105-1111; 2013 (2.0 g, 9.1 mmol) was dissolved in acetonitrile (30.0 mL) and treated with n-bromosuccinimide (1.7 g, 9.5 mmol) and the mixture allowed to stir for 20 hours. The mixture was concentrated in vacuo then filtered through a Si02 plug with hexanes. The solvent was then removed in vacuo to provide an oil (2.6g, 96%) used without further purification. 1H NMR (500 MHz, Chloroform-d) d 7.34 (s, 2H), 3.68 (s, 3H), 1.41 (s, 18H).
References:
WO2020/150668,2020,A1 Location in patent:Paragraph 0191