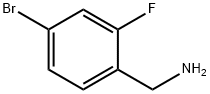
4-BROMO-2-FLUOROBENZYLAMINE synthesis
- Product Name:4-BROMO-2-FLUOROBENZYLAMINE
- CAS Number:112734-22-2
- Molecular formula:C7H7BrFN
- Molecular Weight:204.04
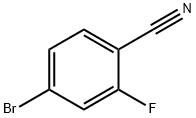
105942-08-3

112734-22-2
General procedure for the synthesis of (4-bromo-2-fluorophenyl)methanamine from 4-bromo-2-fluorobenzonitrile: 4-bromo-2-fluorobenzonitrile (5.00 mmol) was dissolved in tetrahydrofuran (THF, 30 mL) and the solution was cooled to 0°C. A solution of borane tetrahydrofuran complex (1 M solution of THF, 15.0 mL) was added slowly and dropwise with stirring. The reaction mixture was continued to be stirred at 0 °C for 20 min, followed by warming to room temperature and stirring for 16 hours. Upon completion of the reaction, methanol (MeOH, 30 mL) was added slowly and dropwise to quench the reaction, followed by concentration of the solution under reduced pressure. The residue was extracted by partitioning with 1 M aqueous sodium hydroxide (NaOH) and ethyl acetate (EtOAc). The organic layer was separated and post-processed to give (4-bromo-2-fluorophenyl)methylamine (5.00 mmol) as a yellow oil.1H NMR (400 MHz, CDCl3) data: δ 7.42-7.27 (m, 1H), 7.25-7.16 (m, 2H), 3.65 (t, J = 6.6 Hz, 2H).

105942-08-3
382 suppliers
$7.00/5g

112734-22-2
155 suppliers
$9.00/1g
Yield:112734-22-2 5 mmol
Reaction Conditions:
with borane-THF in tetrahydrofuran at 0 - 20; for 16 h;
Steps:
76 (4-Bromo-2-fluoro-phenyl)methanamine
4-Bromo-2-fluorobenzonitrile (5.00 mmol) in THF (30 mL)was cooled to 0 °C. A borane tetrahydrofuran complexsolution (1 M in THF, 15.0 mL) was added dropwise. Thesolution was stirred at 0 °C for 20 mm before being broughtup to RT and stirred for 16 h. MeOH was added dropwise (30 mL) and the solution was concentrated under reduced pressure. The residue was partitionedbetween an aqueous solution of NaOH (1 M) and EtOAc. The organic layer was worked up to give the titled compound (5.00 mmol) as a yellow oil. 1H NMR (400 MHz, CDCI3, ): 7.42-7.27 (m, 1H), 7.25-7.16 (m, 2H), 3.65 (t, J = 6.6 Hz, 2H).
References:
WO2017/103611,2017,A1 Location in patent:Paragraph 00336