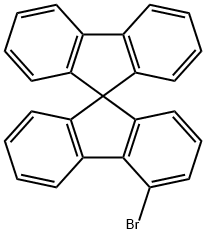
4-bromo-9,9'-Spirobi[9H-fluorene synthesis
- Product Name:4-bromo-9,9'-Spirobi[9H-fluorene
- CAS Number:1161009-88-6
- Molecular formula:C25H15Br
- Molecular Weight:395.29
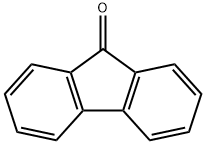
486-25-9
489 suppliers
$5.00/25g
13029-09-9
285 suppliers
$20.00/1g

1161009-88-6
179 suppliers
$7.00/1g
Yield:1161009-88-6 98%
Reaction Conditions:
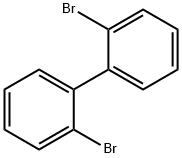
Stage #1:2,2'-dibromobiphenyl with n-butyllithium in tetrahydrofuran;hexane at -78; for 0.5 h;Inert atmosphere;
Stage #2:9-fluorenone in tetrahydrofuran;hexane at -78 - 20;
Stage #3: with hydrogenchloride;acetic acid in tetrahydrofuran;hexane;water for 2 h;Reflux;
Steps:
1.1b 1b) Synthesis of 4-bromo-spiro-9,9'-spirobifluorene
At -78° C under argon with 318mL n-BuLi (in hexane 2.5M, 785mmol)2,2'-dibromobiphenyl(250g, 785mmol) in THF (1900ml) was added.The mixture was stirred for 30 minutes. Was added dropwise 9-one (144g, 785mmol) in 1000mLTHF added. The reaction was carried out at -78° C for 30 minutes and then stirred at room temperature and Stirred overnight. The reaction was quenched with water, and the solid was filtered. Without further purification,Said alcohol (299g, 92%), acetic acid (2200mL) and concentrated HCl (100mL) mixed .It was refluxed for 2 hours. After cooling, the mixture was filtered and washed with water and vacuum dry. The product was isolated as a white solid (280g, 98% of theory).
References:
Merck Patent Ltd. CN105218302, 2016, A Location in patent:Paragraph 0241; 0242; 0243
![9-(2'-bromo-[1,1'-biphenyl]-2-yl)-9H-fluoren-9-ol](/CAS/20200401/GIF/1603849-28-0.gif)
1603849-28-0
1 suppliers
inquiry

1161009-88-6
179 suppliers
$7.00/1g
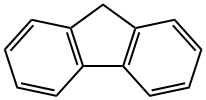
86-73-7
414 suppliers
$6.00/25g

13029-09-9
285 suppliers
$20.00/1g

1161009-88-6
179 suppliers
$7.00/1g
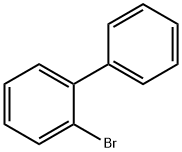
2052-07-5
397 suppliers
$16.30/1g
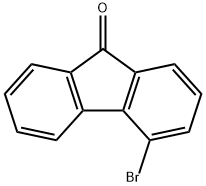
4269-17-4
114 suppliers
$12.00/100mg

1161009-88-6
179 suppliers
$7.00/1g

4269-17-4
114 suppliers
$12.00/100mg
![Lithium, [1,1'-biphenyl]-2-yl-](/CAS/20200119/GIF/55365-18-9.gif)
55365-18-9
1 suppliers
inquiry

1161009-88-6
179 suppliers
$7.00/1g