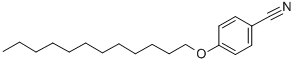
4-DODECYLOXYBENZONITRILE synthesis
- Product Name:4-DODECYLOXYBENZONITRILE
- CAS Number:29147-92-0
- Molecular formula:C19H29NO
- Molecular Weight:287.44
Yield:29147-92-0 93%
Reaction Conditions:
with potassium carbonate in butanone; for 24 h;Reflux;
Steps:
4.5.1. 4-(Dodecyloxy)benzonitrile (3)
Firstly, 15.00 g (126.0 mmol) of 4-hydroxybenzonitrile (1), 42.30 mL (176.0 mmol) of 1-bromododecane, 52.20 g (378.0 mmol) of K2CO3, and 350 mL of butanone were placed into a 500 mL round-bottomed flask equipped with a condenser. The mixture was then refluxed and stirred for 24 h. After this period, the suspension was filtered and washed with hot butanone. The solvent was removed by rotary evaporation and the solid obtained was recrystallized on ethanol, affording 33.60 g of a white crystals (93%), mp 41.5-44.0 °C (lit. 42-43 °C).25 IR (KBr, cm-1): νmax: 2916, 2850, 2218 (CN), 1608, 1509, 1302, 1256, 1172, 833, 547. 1H NMR (CDCl3), δ, ppm: 7.55 (d, J=8.9 Hz, 2H, Ar-H), 6.92 (d, J=8.9 Hz, 2H, Ar-H), 3.98 (t, J=6.6 Hz, 2H, -OCH2-), 1.78 (qt, J=6.6 Hz, 2H, -OCH2CH2-), 1.44 (m, 2H, -CH2-), 1.22-1.33 (br, 16H, -CH2-), 0.87 (t, J=6.7 Hz, 3H, -CH3). 13C NMR (CDCl3), δ, ppm: 162.7, 134.2, 119.5, 115.4, 103.8, 68.6, 32.2, 29.9, 29.9, 14.9.
References:
Gallardo, Hugo;Ferreira, Marli;Vieira, André A.;Westphal, Eduard;Molin, Fernando;Eccher, Juliana;Bechtold, Ivan H. [Tetrahedron,2011,vol. 67,# 49,p. 9491 - 9499] Location in patent:experimental part

4292-19-7
160 suppliers
$18.00/1g
767-00-0
582 suppliers
$6.00/25g
179341-69-6
10 suppliers
inquiry

29147-92-0
10 suppliers
inquiry

