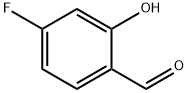
4-FLUORO-2-HYDROXYBENZALDEHYDE synthesis
- Product Name:4-FLUORO-2-HYDROXYBENZALDEHYDE
- CAS Number:348-28-7
- Molecular formula:C7H5FO2
- Molecular Weight:140.11

50-00-0
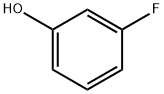
372-20-3

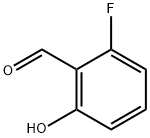
348-28-7
The general procedure for the synthesis of 4-fluoro-2-hydroxybenzaldehyde from formaldehyde and 3-fluorophenol was as follows: to a mixture containing 3-fluorophenol (5 mL, 55.3 mmol) and anhydrous acetonitrile (250 mL) were added MgCl2 (14.2 g, 149 mmol), anhydrous trimethylamine (27 mL) and paraformaldehyde (11 g). The reaction mixture was stirred under reflux conditions for 5 h and subsequently cooled to room temperature. The reaction was quenched by addition of 5% hydrochloric acid (250 mL) and extracted with ethyl acetate (100 mL × 3). The combined organic layers were washed sequentially with 5% hydrochloric acid, water and brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate = 50:1 to 20:1) to afford 4-fluoro-2-hydroxybenzaldehyde (5 g, 65% yield) as a light yellow solid.1H NMR (400 MHz, CDCl3) data were as follows: δ 11.37 (d, J = 1.6 Hz, 1H), 9.83 (s, 1H), 7.55- 7.59 (m, 1H), 6.65-6.75 (m, 2H).

50-00-0
896 suppliers
$10.00/25g

372-20-3
392 suppliers
$7.00/5g

348-28-7
195 suppliers
$21.21/250mgs:
Yield:348-28-7 72%
Reaction Conditions:
with triethylamine;magnesium chloride in acetonitrile for 5 h;Heating / reflux;
Steps:
612.a.1 Example 612a; 7- (ISOBUTYLAMINO)-2- (TRIFLUOROMETHYL)-2H-CHROMENE-3-CARBOXYLIC acid; Step 1. Preparation of 2-HVDROXY-4-FLUOROBENZALDEHYDE.
[1042] To the mixture of 3-fluorophenol (10 mL, 102 mmole), anhydrous magnesium chloride (28.2 g, 744.6 mmole) in 500 mL of anhydrous acetonitrile was added anhydrous triethylamine (67 mL, 382. 5 mmole) and paraformaldehyde (22.3 g, 744.6 mmole). The mixture was then heated to reflux for five hours. After cooling to r. t, 500 mL of 5% aqueous hydrochloric acid was added. The product was extracted with ethyl acetate. The combined organic extracts were washed with 5% hydrochloric acid (x 3), brine, and dried over anhydrous magnesium sulfate. After removing the volatiles, the residue was a light pink solid. 11 g, yield 72%. M. P: 67.5-69. 0°C. ESHRMS 777/Z 139.0211 (M-H, C7H4FO2 calc'd 139. 0201). 1H NMR (CDCl3/300MHz) 11.40 (s, 1H), 9.86 (s, 1H), 7.62-7. 57 (m, 1H), 6.79-6. 67 (m, 2H). 13C (CDC13/300MH) 195.4, 168.3 (d, J= 258 HZ), 164.4 (d, J=14. 9 Hz), 136.3 (d, J=12. 6 Hz), 118.2 (d, J= 2.0Hz), 108.5 (d, J= 23. 3 Hz), 104.9 (d, J=24. 4 HZ). L9F (CDCl3/400MHz) -97. 9 (m).
References:
PHARMACIA CORPORATION WO2004/87686, 2004, A2 Location in patent:Page 504 PHARMACIA CORPORATION WO2004/87687, 2004, A1 Location in patent:Page 504

50-00-0
896 suppliers
$10.00/25g

372-20-3
392 suppliers
$7.00/5g
38226-10-7
174 suppliers
$24.00/1g

348-28-7
195 suppliers
$21.21/250mgs:

372-20-3
392 suppliers
$7.00/5g
100-97-0
0 suppliers
$14.00/250G

348-28-7
195 suppliers
$21.21/250mgs:

372-20-3
392 suppliers
$7.00/5g

75-05-8
1049 suppliers
$10.00/10g

348-28-7
195 suppliers
$21.21/250mgs:
67-66-3
0 suppliers
$33.60/500ml

372-20-3
392 suppliers
$7.00/5g

348-28-7
195 suppliers
$21.21/250mgs: