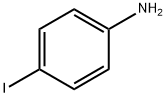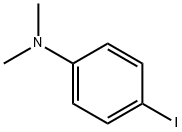
4-Iodo-N,N-dimethyl-Benzenamine synthesis
- Product Name:4-Iodo-N,N-dimethyl-Benzenamine
- CAS Number:698-70-4
- Molecular formula:C8H10IN
- Molecular Weight:247.08
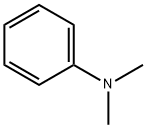
121-69-7

698-70-4
General procedure for the synthesis of 4-N,N-dimethylaminoiodobenzene from N,N-dimethylaniline: to a stirred solution of N,N-dimethylaniline (1 mmol) in dichloromethane (CH2Cl2) or 1,2-dichloroethane ((CH2Cl)2) (0.1 M) was added sequentially Ph3PAuNTf2 (0.025 mmol, 19 mg; if using the 2:1 complex of Ph3PAuNTf2 with toluene) and N-iodosuccinimide (NIS, 1.1 mmol, 248 mg). The reaction mixture was stirred at room temperature or reacted under reflux conditions until complete conversion of the feedstock was shown by thin layer chromatography (TLC) monitoring. Upon completion of the reaction, the solvent was evaporated under reduced pressure and the resulting crude product was purified by fast column chromatography using different ratios of hexane and ethyl acetate (EtOAc) as eluents, resulting in pure 4-N,N-dimethylaminoiodobenzene.
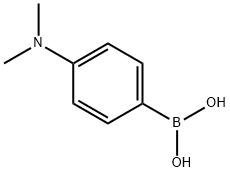
28611-39-4
221 suppliers
$38.43/1G

698-70-4
66 suppliers
$21.00/100mg
Yield: 78%
Reaction Conditions:
with potassium fluoride;iodine in 1,4-dioxane at 80; for 1 h;
Steps:
General procedure
General procedure: A mixture of the arylboronic acid (0.55mmol), KF (96mg, 1.65mmol) and I2 (127mg, 0.50mmol) in 1,4-dioxane (5mL) was stirred at 80°C for 1h. Then it was filtered through silica gel, eluting with Et2O (10mL) and the solvent was removed by rotary evaporation. When necessary, the product was purified by chromatography on silica gel (petroleum ether/Et2O 98:2).
References:
Tramutola, Francesco;Chiummiento, Lucia;Funicello, Maria;Lupattelli, Paolo [Tetrahedron Letters,2015,vol. 56,# 9,p. 1122 - 1123]

121-69-7
565 suppliers
$10.00/25mL

698-70-4
66 suppliers
$21.00/100mg
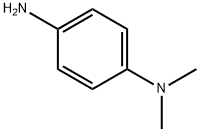
99-98-9
251 suppliers
$16.00/5 g

698-70-4
66 suppliers
$21.00/100mg