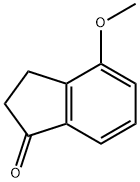
4-METHOXY-1-INDANONE synthesis
- Product Name:4-METHOXY-1-INDANONE
- CAS Number:13336-31-7
- Molecular formula:C10H10O2
- Molecular Weight:162.19
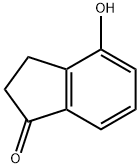
40731-98-4

74-88-4

13336-31-7
The reaction was carried out with 4-hydroxy-1-indanone (125 mg) and iodomethane (263 μL) in the presence of potassium carbonate (350 mg) in N,N-dimethylformamide (DMF, 4 mL) for 3 h at 50 °C with stirring. After completion of the reaction, the mixture was poured into 1N hydrochloric acid (20 mL) and extracted with ether (4 × 10 mL). The organic layers were combined, dried with anhydrous magnesium sulfate, filtered and concentrated to give 4-methoxy-1-indanone intermediate as a clear oil (131 mg, 96% yield). Mass spectral analysis showed [MH]+ = 163.

40731-98-4
237 suppliers
$6.00/1g

74-88-4
357 suppliers
$15.00/10g

13336-31-7
161 suppliers
$5.00/100mg
Yield:13336-31-7 99%
Reaction Conditions:
with potassium carbonate in acetone at 60; for 5 h;
Steps:
1.1a (1a): Preparation of 4-methoxy-1-indanone
4-Hydroxy-1-indanone (2.00 g, 13.5 mmol), potassium carbonate (7.5 g, 54.0 mmol)Add to acetone (40 mL), add methyl iodide (3.8 g, 27.0 mmol), warm to 60 ° C and stir for 5 h.TLC showed the reaction was complete, cooled to room temperature and poured into water.It was extracted twice with ethyl acetate, and the organic phase was washed with brine.Dried over anhydrous sodium sulfate and concentrated to dryness.4-methoxy-1-indanone (2.18 g, yield 99%) was obtained.
References:
Tsingtao Ocean Bio-pharmaceutical Institute;Chen Dong;Xu Tao;Zhao Weifeng;Qin Yuting;Zhang Shouhui;Shan Tiantian CN109776607, 2019, A Location in patent:Paragraph 0030; 0034-0037

67-56-1
790 suppliers
$7.29/5ml-f

40731-98-4
237 suppliers
$6.00/1g

13336-31-7
161 suppliers
$5.00/100mg
6342-77-4
190 suppliers
$9.00/1 g

13336-31-7
161 suppliers
$5.00/100mg

40731-98-4
237 suppliers
$6.00/1g

13336-31-7
161 suppliers
$5.00/100mg

40731-98-4
237 suppliers
$6.00/1g

77-78-1
319 suppliers
$19.69/1ml

13336-31-7
161 suppliers
$5.00/100mg