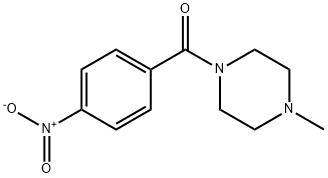
(4-Methylpiperazin-1-yl)(4-nitrophenyl)Methanone synthesis
- Product Name:(4-Methylpiperazin-1-yl)(4-nitrophenyl)Methanone
- CAS Number:21091-98-5
- Molecular formula:C12H15N3O3
- Molecular Weight:249.27
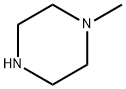
109-01-3
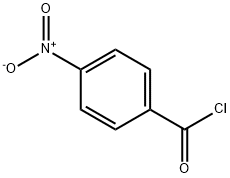
122-04-3

21091-98-5
General procedure for the synthesis of (4-methylpiperazin-1-yl)(4-nitrophenyl)methanone from N-methylpiperazine and 4-nitrobenzoyl chloride: 4-nitrobenzoyl chloride (1 g, 5.39 mmol) was dissolved in acetonitrile (50 mL), cooled to 10 °C and then 1-methylpiperazine was slowly added. The reaction mixture was stirred at this temperature for 10 minutes, followed by continued stirring for 1 hour. Then, triethylamine (1.49 mL, 10.78 mmol) was added and stirring was continued for half an hour. After completion of the reaction, the mixture was diluted with ice-cold water (150 mL) and extracted with ethyl acetate (4 x 150 mL). The organic layers were combined, dried with anhydrous magnesium sulfate and concentrated under reduced pressure to give the yellow solid product (4-methylpiperazin-1-yl)(4-nitrophenyl)methanone (1.1 g, 82% yield). Thin-layer chromatography (TLC) analytical conditions: 10% methanol/dichloromethane, Rf=0.6. Nuclear magnetic resonance hydrogen spectrum (1H NMR, 400MHz, CDCl3) δ: 8.28 (d, J=8.5Hz, 2H), 7.56 (d, J=8.5Hz, 2H), 3.88 (broad single peak, 4H), 3.46 (broad single peak, 4H), 2.40 (s , 3H). Electrospray mass spectrometry (ESI/MS) m/z: 250.0 (M+H).

50-00-0
896 suppliers
$10.00/25g
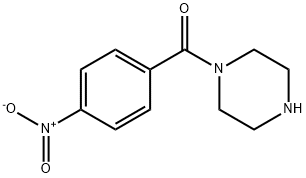
72141-41-4
61 suppliers
inquiry

21091-98-5
35 suppliers
$25.00/250mg
Yield:21091-98-5 900 mg
Reaction Conditions:
with sodium tris(acetoxy)borohydride;triethylamine in dichloromethane at 20; for 3 h;
Steps:
1.1
Step 1’: 1 g of 1’ was converted to 2’ using CH2O/NaBH(OAc)3/TEA/DCM/RT/3h. After purification, 900 mg of 2’ was obtained.
References:
WO2021/236650,2021,A1 Location in patent:Page/Page column 172; 219

109-01-3
673 suppliers
$5.00/5G

122-04-3
39 suppliers
$15.00/1g

21091-98-5
35 suppliers
$25.00/250mg

109-01-3
673 suppliers
$5.00/5G
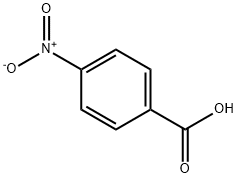
62-23-7
736 suppliers
$14.00/25g

21091-98-5
35 suppliers
$25.00/250mg
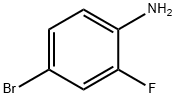
367-24-8
509 suppliers
$15.00/25g
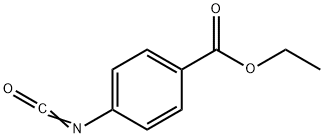
30806-83-8
57 suppliers
$60.00/50mg

21091-98-5
35 suppliers
$25.00/250mg

109-01-3
673 suppliers
$5.00/5G
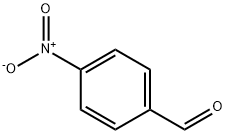
555-16-8
574 suppliers
$5.00/5g

21091-98-5
35 suppliers
$25.00/250mg