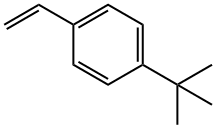
4-TERT-BUTYLSTYRENE synthesis
- Product Name:4-TERT-BUTYLSTYRENE
- CAS Number:1746-23-2
- Molecular formula:C12H16
- Molecular Weight:160.26
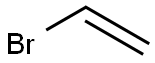
593-60-2
143 suppliers
$25.00/25g
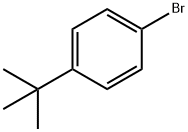
3972-65-4
337 suppliers
$8.00/10g

1746-23-2
125 suppliers
$9.00/25g
Yield:1746-23-2 87%
Reaction Conditions:
with palladium diacetate;C22H27P;triethylamine in 1,4-dioxane;Hexadecane at 120; under 15001.5 Torr; for 20 h;Schlenk technique;Inert atmosphere;Heck Reaction;
Steps:
Experimental procedure for the synthesis of 2-aryl-propionic acids
General procedure: In a 100 mL Schlenk vial 0.75 mol% (11.79 mg) Pd(OAc)2, 3.0 mol% (67.7 mg) NIPCDPP 9 weredissolved in 14 mL dioxane and 0.2 eq (410 μL) hexadecane as internal standard was added. Underargon 2.0 mL of this homogeneous yellow stock solution was transferred to each of six 4 mL vialsequipped with a septum, needle and stirring bar, which are placed in an alloy plate. After 1 mmolof the corresponding aryl bromide and 1.5 eq (208 μL) of NEt3 were added to each vial and a smallsample was withdrawn for GC, the alloy plate was transferred into the 300 mL autoclave. Thesealed autoclave was purged with ethylene several times and pressurized with 10 bar ethylene.Then, the reaction was run at 120 °C for 20 h. Afterwards the autoclave was cooled down to roomtemperature and the gas was carefully released. In the vials a grey precipitate were formed and thesolution was still yellow. After a small sample for GC analyses was withdrawn, 83 μL of 6M HCLwas added carefully. The reaction solution foams. Next, the autoclave was flushed with CO threetimes and the reaction was allowed to run at 40 bar CO. After 20 h, the autoclave was cooled downand the gas was released again. In order to determine the yield by GC, a sample of 100 μL of eachreaction solution was esterified with (trimethylsilyl)diazomethane in the presence of 100 μL MeOH.The products were chromatographed after esterification in 10 mL MeOH and one drop cc H2SO4 (2h refluxing) and characterized by NMR, elemental analysis and mass spectroscopy.
References:
Neumann, Helfried;Sergeev, Alexey G.;Spannenberg, Anke;Beller, Matthias [Molecules,2020,vol. 25,# 15,art. no. 3421] Location in patent:supporting information
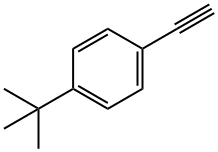
772-38-3
125 suppliers
$29.50/2g

1746-23-2
125 suppliers
$9.00/25g

3972-65-4
337 suppliers
$8.00/10g
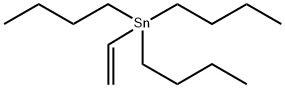
7486-35-3
272 suppliers
$15.00/1g

1746-23-2
125 suppliers
$9.00/25g
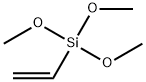
2768-02-7
416 suppliers
$10.00/5g

3972-65-4
337 suppliers
$8.00/10g

1746-23-2
125 suppliers
$9.00/25g
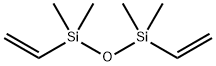
2627-95-4
344 suppliers
$16.80/25G
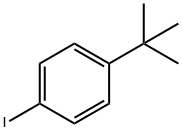
35779-04-5
204 suppliers
$6.00/1g

1746-23-2
125 suppliers
$9.00/25g