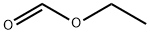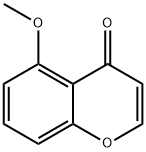
4H-1-Benzopyran-4-one, 5-Methoxy- synthesis
- Product Name:4H-1-Benzopyran-4-one, 5-Methoxy-
- CAS Number:59887-87-5
- Molecular formula:C10H8O3
- Molecular Weight:176.17
Yield:59887-87-5 81%
Reaction Conditions:
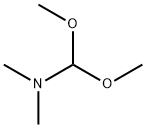
Stage #1: 2'-hydroxy-6'-methoxyacetophenone;formic acid ethyl esterwith sodium hydride at 0; for 3.5 h;Inert atmosphere;
Stage #2: with hydrogenchloride in methanol;water at 0 - 20;Inert atmosphere;
Steps:
4.10. Chromone (1-benzopyran-4-one, 7a)
General procedure: Prepared according to the procedure reported by Li et al.30 Sodium hydride (60% dispersion in mineral oil, 1.06 g, 44.1 mmol, washed three times with hexanes) was added portion-wise to a solution of 2'-hydroxyacetophenone 10a (0.88 mL, 7.34 mmol) in ethyl formate (60 mL) at 0 °C over a period of 2 h. The mixture was allowed to stir for a further 1.5 h, then quenched with methanol (3.5 mL). Concd HCl (11.5 mL) was added, and the mixture allowed to warm to room temperature and stirred for another 16 h. Water (50 mL) was added, and the mixture extracted with dichloromethane (3×50 mL). The combined organic extracts were washed with brine (100 mL), dried over MgSO4, and concentrated under reduced pressure to afford a yellow oil. The crude product was purified via flash column chromatography (silica gel, ethyl acetate/hexanes 1:9 as eluent) to give the title compound 7a (0.990 g, 92%) as pale yellow crystals: mp 52-54 °C (lit.41 55-57 °C).
References:
Stubbing, Louise A.;Li, Freda F.;Furkert, Daniel P.;Caprio, Vittorio E.;Brimble, Margaret A. [Tetrahedron,2012,vol. 68,# 34,p. 6948 - 6956] Location in patent:experimental part
3952-69-0
26 suppliers
$63.61/10mgs:

74-88-4
357 suppliers
$15.00/10g

59887-87-5
10 suppliers
inquiry
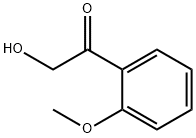
224321-19-1
20 suppliers
inquiry

59887-87-5
10 suppliers
inquiry
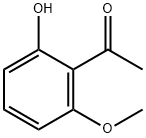
703-23-1
142 suppliers
$21.00/1g

59887-87-5
10 suppliers
inquiry