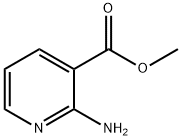
Methyl 2-aminonicotinate synthesis
- Product Name:Methyl 2-aminonicotinate
- CAS Number:14667-47-1
- Molecular formula:C7H8N2O2
- Molecular Weight:152.15

67-56-1
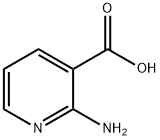
5345-47-1

14667-47-1
Concentrated sulfuric acid (96%, 144 mL, 2.69 mol, 18.6 eq.) was slowly added dropwise to a stirred suspension of 2-aminonicotinic acid (20.0 g, 0.145 mol) in methanol (228 mL, 7.12 mol, 49.2 eq.), and the reaction system was kept at 0 °C. Subsequently, the reaction mixture was irradiated in a microwave reactor (power input 300 W) at 60 °C and atmospheric pressure for 1.5 hours. Upon completion of the reaction, the light brown mixture was carefully poured into ice water, maintained at 0 °C, and solid sodium carbonate was added by batchwise addition until complete neutralization (pH > 8). The aqueous layer was extracted three times with ethyl acetate. The organic layers were combined, washed sequentially with saturated brine and deionized water, and then dried with anhydrous magnesium sulfate. Finally, the organic solvent was evaporated to afford methyl 2-aminopyridine-3-carboxylate (21.15 g, 93% yield) as colorless needle-like crystals with purity meeting the analytical requirements.
Yield:14667-47-1 93%
Reaction Conditions:
with sulfuric acid at 0 - 60; under 760.051 Torr; for 1.5 h;Inert atmosphere;Microwave irradiation;
Steps:
Methyl 2-aminonicotinate (21)
Concentrated sulfuric acid (96%, 144 mL, 2.69 mol, 18.6 equiv) was added dropwise to a stirred suspension of 5 (20.0 g, 0.145 mol) in MeOH (228 mL, 7.12 mol, 49.2 equiv) maintained at 0 C. The mixture was irradiated at atmospheric pressure under microwaves (power input 300 W) at 60 C for 1.5 h. The light brown mixture was poured onto iced water, maintained at 0 C and carefully quenched by adding solid Na2CO3 portionwise until complete neutralization (pH>8). The aqueous layer was extracted three times with AcOEt. The combined organic layers were washed with brine and water and dried over MgSO4. Evaporation of the organic layer yielded the title compound 21 in analytically pure form (21.15 g, 93%) as colourless needles
References:
Hédou, Damien;Deau, Emmanuel;Harari, Marine;Sanselme, Morgane;Fruit, Corinne;Besson, Thierry [Tetrahedron,2014,vol. 70,# 35,p. 5541 - 5549]
1462-86-8
257 suppliers
$10.00/1g

18107-18-1
210 suppliers
$33.00/5g

14667-47-1
213 suppliers
$6.00/1g

5345-47-1
393 suppliers
$9.00/1g

18107-18-1
210 suppliers
$33.00/5g

14667-47-1
213 suppliers
$6.00/1g

5345-47-1
393 suppliers
$9.00/1g
37091-73-9
216 suppliers
$7.00/1g

14667-47-1
213 suppliers
$6.00/1g
