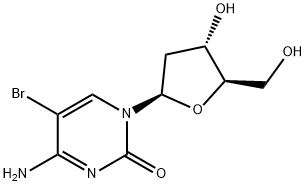
5-Bromo-2'-deoxycytidine synthesis
- Product Name:5-Bromo-2'-deoxycytidine
- CAS Number:1022-79-3
- Molecular formula:C9H12BrN3O4
- Molecular Weight:306.11
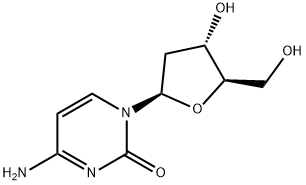
951-77-9

1022-79-3
The general procedure for the synthesis of 4-amino-5-bromo-1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-ones, using 4-amino-1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one as a starting material was carried out in the following manner: under stirring conditions, 2 '-O-methyluridine (5, 0.103 g, 0.4 mmol) was dissolved in aqueous acetonitrile (H2O:CH3CN = 1:9, 5 mL). NaN3 (0.104 g, 1.6 mmol) was then added and SMBI (0.101 g, 0.44 mmol) was added at room temperature. The reaction mixture was stirred continuously and the progress of the reaction was monitored by thin layer chromatography (TLC). The reaction was completed after about 1.5 h. The reaction mixture was filtered, concentrated under reduced pressure and co-evaporated with acetonitrile (2 x 2 mL) to remove residual water. The crude product was purified by column chromatography using a solvent mixture of dichloromethane (DCM) and methanol (MeOH) (4%-6% MeOH in DCM, v/v) as eluent, resulting in purified bromonucleoside 6 (0.117 g, 93% yield) as a white solid.

951-77-9
439 suppliers
$5.00/100mg

1022-79-3
135 suppliers
$37.00/250mg
Yield: 90%
Reaction Conditions:
with N-Bromosuccinimide;acetic acid at 20; for 0.333333 h;
References:
Kanamori, Takashi;Masaki, Yoshiaki;Mizuta, Masahiro;Tsunoda, Hirosuke;Ohkubo, Akihiro;Sekine, Mitsuo;Seio, Kohji [Organic and Biomolecular Chemistry,2012,vol. 10,# 5,p. 1007 - 1013] Location in patent:supporting information; experimental part

951-77-9
439 suppliers
$5.00/100mg

1022-79-3
135 suppliers
$37.00/250mg
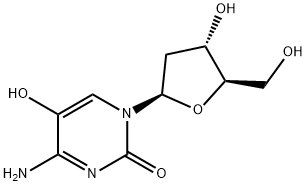
52278-77-0
24 suppliers
$40.00/2 mg
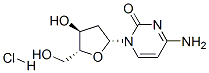
3992-42-5
240 suppliers
$20.00/1g

1022-79-3
135 suppliers
$37.00/250mg