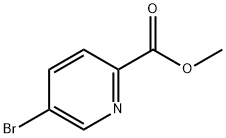
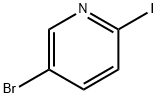
5-BROMOPYRIDINE-2-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:5-BROMOPYRIDINE-2-CARBOXYLIC ACID METHYL ESTER
- CAS Number:29682-15-3
- Molecular formula:C7H6BrNO2
- Molecular Weight:216.03

67-56-1
30766-11-1

29682-15-3
Example 89 - Preparation of Intermediate 25: The synthesis of Intermediate 25 followed the following general procedure. To a solution of 5-bromopyridine-2-carboxylic acid (50.0 g, 0.247 mol, 1.0 eq.) in anhydrous methanol (400 mL) cooled to 0 °C was added thionyl chloride (107.0 mL, 2.47 mol, 10.0 eq.) dropwise. The reaction mixture was slowly warmed to room temperature and subsequently heated at 50 °C for 12 hours. The reaction process was monitored by thin layer chromatography (TLC) and liquid chromatography-mass spectrometry (LC-MS). After completion of the reaction, the reaction mixture was concentrated under reduced pressure to give a white solid residue. The residue was slowly quenched with saturated sodium bicarbonate solution and the white solid was collected by filtration to afford the target product methyl 5-bromopyridine-2-carboxylate (43.0 g, 80% yield). The product was characterized by mass spectrometry (m/z 216.14) and nuclear magnetic resonance hydrogen spectroscopy (1H NMR, 400 MHz, DMSO): δ 8.86 (d, J=1.9 Hz, 1H), 8.28 (dd, J=8.4,2.4 Hz, 1H), 8.00 (d, J=8.4 Hz, 1H), 3.89 (s, 3H) ppm.

67-56-1
790 suppliers
$7.29/5ml-f

30766-11-1
406 suppliers
$3.00/1g

29682-15-3
285 suppliers
$8.00/1g
Yield:29682-15-3 92%
Reaction Conditions:
with sulfuric acid at 0 - 70; for 6 h;
Steps:
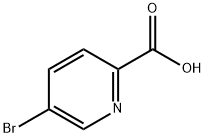
12 Synthesis of methyl 5-bromopicolinate (12.5).
To a solution of 5-bromopicolinic acid (12.6, 6.0 g, 29.9 mmol) in methanol (60 mL), sulfuric acid (3.0 mL) was added at 0 °C and the reaction mixture was heated at 70 °C for 6 h. After completion, cooled the mixture to room temperature and methanol was removed under reduced pressure. To the mixture, water (50 mL) was added and pH was adjusted to 6 by a solution of sodium bicarbonate .The product was extracted with ethyl acetate (200 mL), the organic layer was dried over anhydrous sodium sulphate and concentrated under reduced pressure. The crude product was purified by washings with diethyl ether and pentane to afford methyl 5-bromopicolinate (12.5) as white solid. Yield: 6.0 g, 92.0%.
References:
ADHAERE PHARMACEUTICALS, INC.;BARBOSA, Antonio J. WO2018/126072, 2018, A1 Location in patent:Paragraph 0246; 0247
624-28-2
720 suppliers
$5.00/5g

67-56-1
790 suppliers
$7.29/5ml-f
201230-82-2
1 suppliers
inquiry

29682-15-3
285 suppliers
$8.00/1g

30766-11-1
406 suppliers
$3.00/1g
18107-18-1
210 suppliers
$33.00/5g

29682-15-3
285 suppliers
$8.00/1g

624-28-2
720 suppliers
$5.00/5g
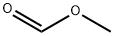
107-31-3
356 suppliers
$16.00/25mL

29682-15-3
285 suppliers
$8.00/1g

67-56-1
790 suppliers
$7.29/5ml-f
201230-82-2
1 suppliers
inquiry
223463-13-6
382 suppliers
$5.00/1g

29682-15-3
285 suppliers
$8.00/1g