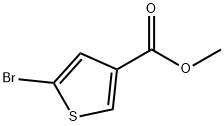
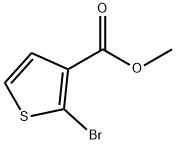
Methyl 5-broMothiophene-3-carboxylate synthesis
- Product Name:Methyl 5-broMothiophene-3-carboxylate
- CAS Number:88770-19-8
- Molecular formula:C6H5BrO2S
- Molecular Weight:221.07

67-56-1
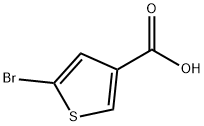
100523-84-0

88770-19-8
1. Add a solution of bromine (2 mL, 39.06 mmol) in glacial acetic acid (64 mL) slowly dropwise to a stirred solution of 5-bromothiophene-3-carboxylic acid (5 g, 39.06 mmol) in glacial acetic acid (37 mL) at 0 °C. Stirring was done for 20 minutes at room temperature. The reaction mixture was poured into ice water with vigorous stirring. The white solid precipitate was collected by filtration, washed with water and recrystallized from hot water to give pure 5-bromothiophene-3-carboxylic acid bromide (Int-2) as a white solid (3.68 g, 46%).1H NMR (200 MHz, DMSO-d6): δ 7.55 (s, 1H), 8.17 (s, 1H). 2. Int-2 (3.4 g, 16.42 mmol) was dissolved in methanol (40 mL), H2SO4 (321 mg, 3.28 mmol) was added, and stirred at reflux for 12 hours. Volatiles were removed by distillation under reduced pressure and the residue was extracted with DCM (75 mL). The organic phase was washed sequentially with water (40 mL), saturated aqueous NaHCO3 (40 mL), brine (40 mL), dried over Na2SO4, filtered and concentrated under reduced pressure to give pure methyl 5-bromothiophene-3-carboxylate (Int-3) as a colorless viscous oil (3.3 g, 91%).1H NMR (200 MHz, DMSO-d6): δ 7.98 (s, 1H), 7.44 (s, 1H), 3.87 (s, 3H). 3. Int-3 (0.5 g, 2.26 mmol) was dissolved in dioxane (25 mL), and Int-4 (0.51 g, 2.26 mmol), Pd(OAc)2 (105 mg, 0.45 mmol), xanthic acid (265 mg, 0.497 mmol) and Cs2CO3 (1.18 g, 3.62 mmol) were added. After vacuum degassing, the reaction was stirred at reflux for 20 h under N2 protection. The reaction mixture was concentrated under reduced pressure and purified by column chromatography with the product eluting with EtOAc. The mixture was further purified by preparative HPLC (140 mg) to give pure Int-5 as a yellow solid (80 mg, 10%). Mass spectrum (m/z): 366.0 [M++1]. 1H NMR (200 MHz, DMSO-d6): δ 9.50 (d, J=7 Hz, 1H), 8.51 (d, J=7 Hz, 1H), 8.56 (d, J=5.2 Hz, 1H), 7.74 (brs, 1H), 7.66 (d, J=11.8 Hz, 1H). 7.35 (d, J=7.4 Hz, 1H), 7.11 (s, 1H), 7.02 (d, J=5.6 Hz, 1H), 6.87 (t, J=6.8 Hz, 1H), 3.86 (s, 3H), 2.73 (s, 3H). 4. Int-5 (0.7 g, 1.91 mmol) was dissolved in THF:MeOH:H2O (2:1:2, 50 mL), LiOH (0.24 g, 5.74 mmol) was added, and heated and stirred at 50 °C for 16 hours. Volatiles were removed by distillation under reduced pressure and the reaction mixture was acidified to pH 5-6 with 2N HCl. The precipitated solid was filtered and dried under vacuum to give pure Int-6 (0.51 g, 76%). Mass spectrum (m/z): 352.0 [M++1]. 1H NMR (200 MHz, DMSO-d6): δ 12.45 (brs, 1H), 10.87 (s, 1H), 9.70 (brs, 1H), 8.60 (d, J=4.68 Hz, 1H), 7.67-7.59 (m, 2H), 7.45 (t, J=8.4 Hz, 1H), 7.13 (d, J=5.2 Hz, 1H), 7.04 (s, 2H), 2.65 (s, 3H).

67-56-1
790 suppliers
$7.29/5ml-f

100523-84-0
177 suppliers
$8.00/250mg

88770-19-8
81 suppliers
$31.00/250mg
Yield: 91%
Reaction Conditions:
Stage #1:methanol;2-bromothiophene-4-carboxylic acid with sulfuric acid for 12 h;Reflux;
Stage #2: with sodium hydrogencarbonate in water
Steps:
59; 60
A solution of bromine (2 mL, 39.06 mmol) in glacial acetic acid (64 mL) was added slowly to a stirred solution of Int-1 (5 g, 39.06 mmol) in glacial acetic acid (37 mL) at 0° C. and stirred at room temperature for 20 minutes. The reaction mixture was then poured into ice water while stirring vigorously. The white precipitated solid was filtered, washed with water and recrystallised from hot water to afford pure Int-2 as white solid (3.68 g, 46%). 1H NMR (200 MHz, dmso-d6): δ 7.55 (s, 1H), 8.17 (s, 1H). To a stirred solution of Int-2 (3.4 g, 16.42 mmol) in methanol (40 mL) was added H2SO4 (321 mg, 3.28 mmol) and stirred under reflux for 12 hours. Volatiles from the reaction mixture were distilled off under reduced pressure and the resulting residue was extracted with DCM (75 mL). The organic extract was washed with water (40 mL), saturated aqueous NaHCO3 solution (40 mL), brine (40 mL) and dried over Na2SO4, filtered and concentrated under vacuum to afford pure Int-3 as colorless viscous oil (3.3 g, 91%). 1H NMR (200 MHz, dmso-d6): δ 7.98 (s, 1H), 7.44 (s, 1H), 3.87 (s, 3H). To a stirred solution of Int-3 (0.5 g, 2.26 mmol) in dioxane (25 mL) was added Int-4 (0.51 g, 2.26 mmol) followed by Pd (OAc) 2 (105 mg, 0.45 mmol), xanthpos (265 mg, 0.497 mmol) and CS2CO3 (1.18 g, 3.62 mmol). The reaction mixture was degassed under vacuum, bubbled with N2 for 10 minutes and stirred under reflux for 20 hours. The reaction mixture was concentrated under reduced pressure and was purified by column chromatography to isolate product and a very close spot together eluting with EtOAc. This mixture (140 mg) was further purified with preparative HPLC to obtain pure Int-5 as a yellow solid (80 mg, 10%). Mass (m/z): 366.0 [M++1]. 1H NMR (200 MHz, dmso-d6): 9.50 (d, J=7 Hz, 1H), 8.51 (d, J=7 Hz, 1H), 8.56 (d, J=5.2 Hz, 1H), 7.74 (brs, 1H), 7.66 (d, J=11.8 Hz, 1H), 7.35 (d, J=7.4 Hz, 1H), 7.11 (s, 1H), 7.02 (d, J=5.6 Hz, 1H), 6.87 (t, J=6.8 Hz, 1H), 3.86 (s, 3H), 2.73 (s, 3H). To a stirred solution of Int-5 (0.7 g, 1.91 mmol) in THF: MeOH: H2O (2:1:2) (50 ml) was added LiOH (0.24 g, 5.74 mmol) at room temperature and stirred while heating at 50° C. for 16 hours. Volatiles from the reaction mixture were distilled off under reduced pressure, the reaction mixture was acidified to about pH 5-6 with 2N HCl. The precipitated solid was filtered and dried under vacuum to afford pure Int-6 (0.51 g, 76%). Mass (m/z): 352.0 [M++1]. 1H NMR (200 MHz, dmso-d6): δ 12.45 (brs, 1H), 10.87 (s, 1H), 9.70 ((brs, 1H), 8.60 (d, J=4.68 Hz, 1H), 7.67-7.59 (m, 2H), 7.45 (t, J=8.4 Hz, 1H), 7.13 (d, J=5.2 Hz, 1H), 7.04 (s, 2H), 2.65 (s, 3H).
References:
Melvin, JR., Lawrence S.;Graupe, Michael;Venkataramani, Chandrasekar US2010/29638, 2010, A1 Location in patent:Page/Page column 110

100523-84-0
177 suppliers
$8.00/250mg

77-78-1
319 suppliers
$19.69/1ml

88770-19-8
81 suppliers
$31.00/250mg
88-13-1
375 suppliers
$5.00/1g

88770-19-8
81 suppliers
$31.00/250mg
89280-91-1
36 suppliers
inquiry
76360-43-5
103 suppliers
$33.00/250mg

88770-19-8
81 suppliers
$31.00/250mg

100523-84-0
177 suppliers
$8.00/250mg

88770-19-8
81 suppliers
$31.00/250mg