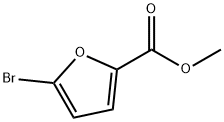
METHYL 5-BROMO-2-FUROATE synthesis
- Product Name:METHYL 5-BROMO-2-FUROATE
- CAS Number:2527-99-3
- Molecular formula:C6H5BrO3
- Molecular Weight:205.01

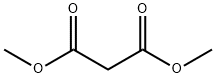
67-56-1
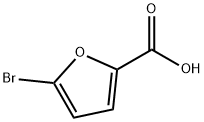
585-70-6

2527-99-3
General procedure for the synthesis of methyl 5-bromofuran-2-carboxylate from methanol and 5-bromo-2-furoic acid: 5-bromo-2-furoic acid (950 mg, 5.00 mmol) was accurately weighed and dissolved in 10 mL of methanol, and 2 mL of sulfoxide chloride (SOCl2) was added slowly and dropwise. Upon completion of the reaction, the reaction mixture was cooled to room temperature and the solvent was subsequently removed by evaporation under reduced pressure. The residue was dissolved in an appropriate amount of toluene and this dissolution and evaporation process was repeated three times to give the intermediate 5-bromo-2-furoic acid methyl ester in 100% yield.
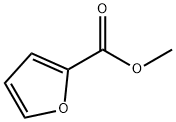
611-13-2
436 suppliers
$13.00/25g

2527-99-3
189 suppliers
$6.00/1g
Yield:2527-99-3 85%
Reaction Conditions:
with bromine at 50; for 0.5 h;Inert atmosphere;
Steps:
Methyl 5-bromo-2-furoate S6
Prepared according to the approach noted in Torii, S. et al., Anodic Reaction of 2-Furoic Acids. II. Electrolysis of Methyl 5-Acetyl-2-furoate and Its Homologous in Protic Solvents. Bull. Chem. Soc. Jpn. 1972, 45 (9), 2783-2787. DOI: 10.1246/bcsj.45.2783, the entire content of which is incorporated herein by reference. Bromine (6.07 g, 0.038 mol) was carefully added (dropwise over a period of 15 minutes) to a solution of methyl furoate (S5, 3.2 g, 0.025 mol) stirred at 50° C. under an argon atmosphere in a flame-dried round-bottom flask. The resulting dark orange/brownish solution was additionally stirred for another 15 minutes at 50° C. The reaction mixture was then poured into cold water (10 mL) and extracted with ethyl acetate (2×50 mL). The combined extracts were washed with water (1×10 mL) and brine (1×10 mL) prior to being dried with magnesium sulfate and concentrated. The final product was purified by flash chromatography (10:1, hexanes-ethyl acetate) to obtain 4.5 g of S6 in 85% yield. The spectral data was consistent with literature reports as noted in Torii above. Rf=0.17 (7:3, hexanes-ethyl acetate).
References:
University of Windsor US2021/40105, 2021, A1 Location in patent:Paragraph 0096

74-88-4
357 suppliers
$15.00/10g

2527-99-3
189 suppliers
$6.00/1g