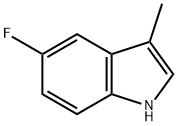
5-Fluoro-3-methylindole synthesis
- Product Name:5-Fluoro-3-methylindole
- CAS Number:392-13-2
- Molecular formula:C9H8FN
- Molecular Weight:149.16
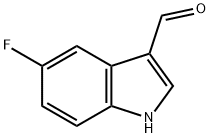
2338-71-8

392-13-2
5-Fluoro-1H-indole-3-carboxaldehyde (3.2 g, 19.6 mmol) was used as raw material and dissolved in 10 mL of anhydrous tetrahydrofuran. Subsequently, lithium aluminum hydride (2.61 g, 68.6 mmol) was added to this solution. The reaction mixture was stirred at 70 °C for 2 hours. Upon completion of the reaction, the mixture was cooled to room temperature and the reaction was quenched with 18 mL of 20% sodium hydroxide aqueous solution. The mixture was extracted by adding 60 mL of dichloromethane to the mixture and the organic layer was washed with 40 mL of saturated aqueous sodium chloride solution. The organic phase was dried over anhydrous sodium sulfate and filtered, and the filtrate was concentrated under reduced pressure. The residue was purified by silica gel column chromatography with petroleum ether/ethyl acetate (V/V=50/1) as eluent to afford 5-fluoro-3-methylindole as a light yellow solid (2.41 g, 82.3% yield). The structure of the compound was confirmed by the following spectral data: mass spectrum (ESI, positive ion mode) m/z: 150.2 [M+H]+; 1H NMR (400 MHz, CDCl3) δ: 7.30-7.23 (m, 2H), 7.04 (s, 1H), 6.97 (td, J=9.0,2.0 Hz, 1H), 2.33 (s, 3H).

2338-71-8
168 suppliers
$15.00/250mg

392-13-2
127 suppliers
$32.00/100mg
Yield: 82.3%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 70; for 2 h;
Steps:
58.1 Step 1) Synthesis of 5-fluoro-3-methyl-1H-indole
5-Fluoro-1H-indole-3-carbaldehyde (3.2 g, 19.6 mmol) was dissolved in 10 mL of anhydrous tetrahydrofuran, then lithium aluminium hydride (2.61 g, 68.6 mmol) was added to the solution. The mixture was reacted at 70 °C for 2 hours. After the reaction, the reaction mixture was cooled to RT and quenched with aqueous sodium hydroxide (18 mL, 2 0%). 60 mL of dichloromethane was added to the mixture and the resulting mixture was washed with saturated aqueous sodium chloride (40 mL). The organic layer was dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated in vacuo and the residue was purified by silica gel chromatography eluted with PE/EA(V/V)=50/1 to give the title compound as a light yellow solid (2.41 g, 82.3%). The compound was characterized by the following spectroscopic data: MS (ESI, pos. ion) m/z:150.2 [M+Hfb; and ‘H NMR (400 MHz, CDC13): 6 7.30-7.23 (m, 2H), 7.04 (s, 1H), 6.97 (td, J = 9.0, 2.0 Hz, 1H), 2.33 (s, 3H).
References:
SUNSHINE LAKE PHARMA CO., LTD.;ZHANG, Yingjun;JIN, Chuanfei;ZHONG, Wenhe;ZHANG, Ji;GAO, Li;CHEN, Kangzhi WO2015/90233, 2015, A1 Location in patent:Paragraph 00362

67-56-1
790 suppliers
$7.29/5ml-f
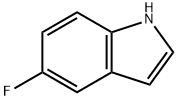
399-52-0
383 suppliers
$10.00/1g

392-13-2
127 suppliers
$32.00/100mg

399-52-0
383 suppliers
$10.00/1g

392-13-2
127 suppliers
$32.00/100mg
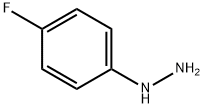
371-14-2
81 suppliers
inquiry

392-13-2
127 suppliers
$32.00/100mg
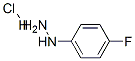
823-85-8
366 suppliers
$5.00/5g
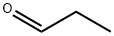
123-38-6
455 suppliers
$14.00/5mL

392-13-2
127 suppliers
$32.00/100mg