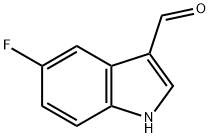
5-FLUOROINDOLE-3-CARBOXALDEHYDE synthesis
- Product Name:5-FLUOROINDOLE-3-CARBOXALDEHYDE
- CAS Number:2338-71-8
- Molecular formula:C9H6FNO
- Molecular Weight:163.15
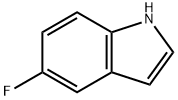
399-52-0

68-12-2

2338-71-8
GENERAL METHOD: Phosphoryl chloride (0.42 g, 2.74 mmol) was slowly added dropwise to a solution of N,N-dimethylformamide (0.84 g, 11.4 mmol) of 5-fluoroindole (0.30 g, 2.29 mmol) at 0 °C, the dropwise process lasted for 30 min. Subsequently, the reaction mixture was warmed up to 40 °C and kept for 1 hour. Upon completion of the reaction, ice was added to the reaction vessel followed by 2 M sodium hydroxide solution. The mixture was refluxed for 40 minutes. After the reaction solution was cooled to room temperature, it was extracted with ethyl acetate. The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography with the eluent being a mixed solvent of petroleum ether-ethyl acetate to give the target compound 5-fluoroindole-3-carboxaldehyde [19].

399-52-0
383 suppliers
$10.00/1g
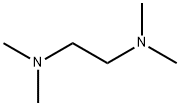
110-18-9
570 suppliers
$10.00/5mL

2338-71-8
168 suppliers
$15.00/250mg
Yield: 76%
Reaction Conditions:
with water;iodine;oxygen;sodium carbonate in 1,4-dioxane at 100; under 760.051 Torr; for 36 h;Schlenk technique;Sealed tube;
Steps:
Experimental Procedures for the C3-Formylation of Indoles
General procedure: Under air, a 20 mL of Schlenk tube equipped with a stir bar was charged with indole 1 (0.2 mmol, 1 equiv),TMEDA (75 µL, 0.5 mmol, 2.5 equiv), Na2CO3 (42.4 mg, 0.4mmol, 2.0 equiv), 1,4-dioxane (0.5 mL) and H2O (100 µL). Then I2 (101.5 mg, 0.4 mmol, 2.0 equiv) was added and the tube was sealed with a rubber plug and charged with O2. The reaction mixture was stirred at 100 °C for 36 h in oil bath. After cooling to room temperature, the resultant mixture was evaporated with EtOAc (20 mL) under reduced pressure and the residue was purified by flash column chromatography on a silica gel to give the products.
References:
Zhang, Bo;Liu, Bin;Chen, Jianbin;Wang, Jiehui;Liu, Miaochang [Tetrahedron Letters,2014,vol. 55,# 41,p. 5618 - 5621] Location in patent:supporting information

399-52-0
383 suppliers
$10.00/1g
100-97-0
0 suppliers
$14.00/250G

2338-71-8
168 suppliers
$15.00/250mg

399-52-0
383 suppliers
$10.00/1g

50-00-0
896 suppliers
$10.00/25g

2338-71-8
168 suppliers
$15.00/250mg

399-52-0
383 suppliers
$10.00/1g
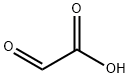
298-12-4
476 suppliers
$5.00/25g

2338-71-8
168 suppliers
$15.00/250mg

399-52-0
383 suppliers
$10.00/1g
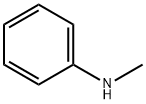
100-61-8
401 suppliers
$8.00/1g

2338-71-8
168 suppliers
$15.00/250mg