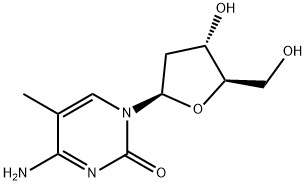
5-Methyl-2'-deoxycytidine synthesis
- Product Name:5-Methyl-2'-deoxycytidine
- CAS Number:838-07-3
- Molecular formula:C10H15N3O4
- Molecular Weight:241.24
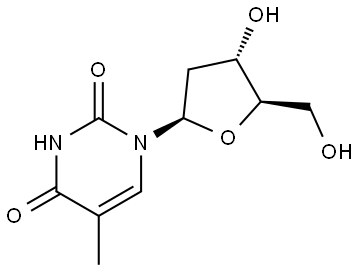
50-89-5

838-07-3
The general procedure for the synthesis of 4-amino-1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidin-2,4(1H,3H)-diones, using 1-((2R,4S,5R)-4-hydroxy-5-(hydroxymethyl)tetrahydrofuran-2-yl)-5-methylpyrimidin-2(1H)-one as a starting material was carried out as follows. DMF a nucleoside solution was prepared (substrate concentration of 0.3 M, except for compounds 3 and 4, which was 0.05 M) to which PyAOP (1.6 eq.) and DBU (1.6 eq.) were added. The reaction mixture was stirred at 20 °C for 1 min. Subsequently, N-nucleophilic reagent (4 eq. dissolved in THF if 1a was used, or concentrated NH4OH, 10 eq.) was added to the reaction system. The reaction continued to be stirred at 20 °C for 5 min to 4 h and the progress of the reaction was monitored by TLC. Upon completion of the reaction, the solution was concentrated under vacuum. Separation by fast column chromatography on silica gel afforded purified forms of the target product 5MedC and its oxidized derivatives 1'-20'.

50-89-5
657 suppliers
$9.00/1g

838-07-3
269 suppliers
$9.00/100mg
Yield:838-07-3 92%
Reaction Conditions:
Stage #1: thymidinewith 1,8-diazabicyclo[5.4.0]undec-7-ene;((3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl)oxy)tri(pyrrolidin-1-yl)phosphonium hexafluorophosphate(V) in N,N-dimethyl-formamide at 20; for 0.0166667 h;
Stage #2: with ammonium hydroxide in N,N-dimethyl-formamide at 20; for 2 h;Reagent/catalyst;Time;
Steps:
General procedure for the synthesis of 5MedC and oxidized 5MedC derivatives (1′-20′)
General procedure: To a solution of the nucleosides (2-20) in DMF ([substrate]=0.3M, except for 3 and 4, [3,4]=0.05M) were added PyAOP (1.6 eq) and DBU (1.6 eq). The reaction was stirred at 20°C for 1min. To the reaction solution was added N-nucleophiles (4 eq) (or 1a in THF, conc. NH4OH (10 eq)). The reaction was stirred at 20°C for 5min-4h and monitored by TLC. Upon completion, the solution was concentrated in vacuo. Flash column chromatography on silica gel afforded 5MedC and oxidized 5MedC derivatives (1′-20′) in pure form.
References:
Zheng, Xiu-An;Huang, Hua-Shan;Kong, Rui;Chen, Wei-Jie;Gong, Shan-Shan;Sun, Qi [Tetrahedron,2018,vol. 74,# 49,p. 7095 - 7101]
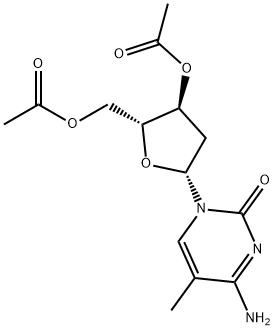
115652-25-0
1 suppliers
inquiry

838-07-3
269 suppliers
$9.00/100mg
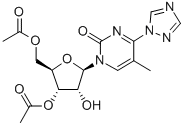
80991-41-9
8 suppliers
inquiry

838-07-3
269 suppliers
$9.00/100mg
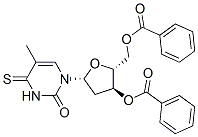
93841-35-1
1 suppliers
inquiry

838-07-3
269 suppliers
$9.00/100mg
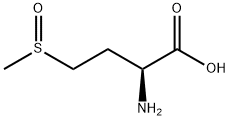
3226-65-1
174 suppliers
$9.00/1g
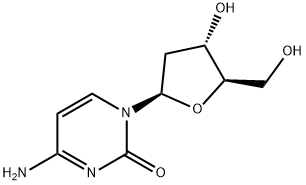
951-77-9
439 suppliers
$5.00/100mg

838-07-3
269 suppliers
$9.00/100mg