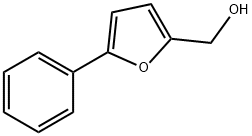
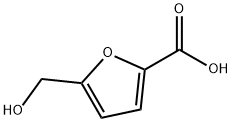
(5-PHENYL-FURAN-2-YL)-METHANOL synthesis
- Product Name:(5-PHENYL-FURAN-2-YL)-METHANOL
- CAS Number:22078-90-6
- Molecular formula:C11H10O2
- Molecular Weight:174.2
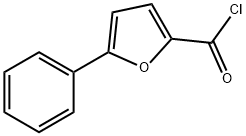
57489-93-7
19 suppliers
$81.37/1g

22078-90-6
28 suppliers
$45.00/10mg
Yield:22078-90-6 80%
Reaction Conditions:
with sodium tetrahydroborate in tetrahydrofuran;N,N-dimethyl-formamide at -10 - 0; for 2 h;Inert atmosphere;
Steps:
2.2.1. General procedure for the synthesis of title compounds 3a-3k
General procedure: According to the reported literature [10aec], Intermediates 5-substituted phenyl-2-furoic acid 7a-7k were prepared fromsubstituted aniline by Meerwein arylation reaction, and the corresponding5-phenylfuran-2-carbonyl chloride were prepare usingSOCl2 as solvent and reactant. A solution of 5-phenylfuran-2-carbonyl chloride (5.0 mmol, 1 equiv) was prepared in dry THFand cooled to 10 C under nitrogen atmosphere. Sodium borohydride(6.0 mmol, 1.2 equiv) was added to the THF solution andthe reaction mixture was stirred for 2 h at 0 C. The reaction wasquenched by adding 10% aqueous ammonium chloride solution.THF was distilled off and the residue was diluted by adding chloroformand water. The product was extracted with three portions ofchloroform and the organic layers were combined, dried overanhydrous MgSO4, filtered and concentrated to get the crudealcohol. It was purified by column chromatography (silica gel,hexane and chloroform as eluents) to isolate a colorless liquid (80%yield).
References:
Lin, Yinuo;Ahmed, Wasim;He, Min;Xiang, Xuwen;Tang, Riyuan;Cui, Zi-Ning [European Journal of Medicinal Chemistry,2020,vol. 207]
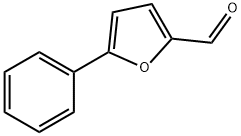
13803-39-9
110 suppliers
$39.00/250mg

22078-90-6
28 suppliers
$45.00/10mg
98-00-0
489 suppliers
$23.00/50g
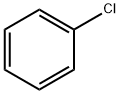
108-90-7
685 suppliers
$10.00/25G

22078-90-6
28 suppliers
$45.00/10mg
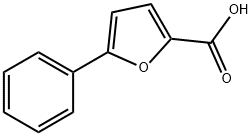
52938-97-3
88 suppliers
$45.00/10mg

22078-90-6
28 suppliers
$45.00/10mg
6338-41-6
209 suppliers
$5.00/100mg

108-86-1
523 suppliers
$10.00/5g

22078-90-6
28 suppliers
$45.00/10mg