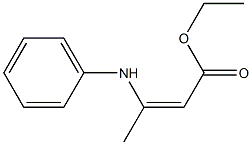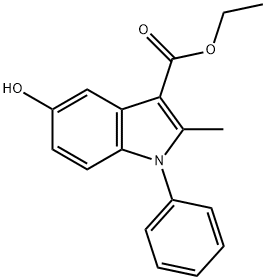
5-HYDROXY-2-METHYL-1-PHENYL-1H-INDOLE-3-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:5-HYDROXY-2-METHYL-1-PHENYL-1H-INDOLE-3-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:5564-29-4
- Molecular formula:C18H17NO3
- Molecular Weight:295.33
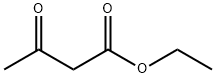
141-97-9
825 suppliers
$5.00/25g
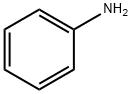
62-53-3
688 suppliers
$10.00/1g
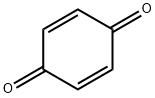
106-51-4
494 suppliers
$10.00/5g

5564-29-4
10 suppliers
$467.00/1g
Yield:5564-29-4 96%
Reaction Conditions:
Stage #1: ethyl 3-oxobutanoate;anilinewith montmorillonite KSF clay in 1,2-dichloro-ethane; for 0.25 h;Reflux;Green chemistry;Nenitzescu Synthesis;
Stage #2: p-benzoquinone in 1,2-dichloro-ethane; for 1 h;Reflux;Green chemistry;Nenitzescu Synthesis;
Steps:
Representative Procedure for the Synthesis of 5-Hydroxyindole Derivatives.
General procedure: A mixture of aniline (1 mmol),dimedone (1 mmol) and montmorillonite KSF clay (0.5 g) in1,2-dichloroethane (5 mL) was kept under reflux for 15 min. To this mixture,1,4-benzoquinone (1 mmol) was added and the resulting mixture was allowed to stir under reflux for the appropriate time (Table 1). After completion of the reaction as indicated by TLC, the mixture was filtered and washed with dichloromethane (10 mL). The combined organic layers were concentrated in vacuo and the resulting residue was purified by column chromatography on silica gel (Merck, 60-120 mesh, ethyl acetate-hexane,1:9) to afford the pure 5-hydroxyindole derivative.
References:
Reddy, B.V.Subba;Reddy, P. Sivaramakrishna;Reddy, Y. Jayasudhan;Bhaskar;Reddy, B. Chandra Obula [Bulletin of the Korean Chemical Society,2013,vol. 34,# 10,p. 2968 - 2972]
591-50-4
500 suppliers
$10.00/1g
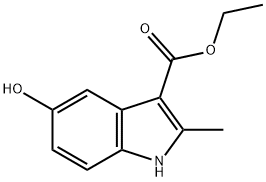
7598-91-6
106 suppliers
$60.00/50mg

5564-29-4
10 suppliers
$467.00/1g
6287-35-0
67 suppliers
$28.60/250mg

106-51-4
494 suppliers
$10.00/5g

5564-29-4
10 suppliers
$467.00/1g

141-97-9
825 suppliers
$5.00/25g

62-53-3
688 suppliers
$10.00/1g
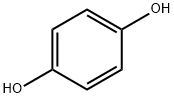
123-31-9
864 suppliers
$13.00/25g

5564-29-4
10 suppliers
$467.00/1g