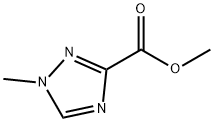
1H-1,2,4-Triazole-3-carboxylicacid,1-methyl-,methylester(9CI) synthesis
- Product Name:1H-1,2,4-Triazole-3-carboxylicacid,1-methyl-,methylester(9CI)
- CAS Number:57031-66-0
- Molecular formula:C5H7N3O2
- Molecular Weight:141.13
4928-88-5

74-88-4

57031-66-0
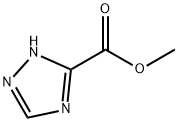
Step A: Preparation of methyl 1-methyl-1H-1,2,4-triazole-3-carboxylate: To a suspension of sodium hydride (60% oil dispersion, 0.346 g, 8.66 mmol) in N,N-dimethylformamide (DMF, 20 mL) under stirring at 0°C under nitrogen protection was slowly added dropwise methyl 1H-1,2,4-triazole-3-carboxylate (1.00 g, a 7.87 mmol) solution in DMF (20 mL). The reaction mixture was continued to be stirred at 0 °C for 1 hour. Subsequently, iodomethane (MeI, 0.982 mL, 15.7 mmol) was added dropwise. The reaction mixture was stirred at room temperature overnight. Upon completion of the reaction, the mixture was poured into cold water and extracted with ethyl acetate (EtOAc). The organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by silica gel column chromatography (eluent: hexane/ethyl acetate=3:1) to afford the target product methyl 1-methyl-1H-1,2,4-triazole-3-carboxylate (0.380 g, 34% yield) as a white solid. Mass spectrum (APCI) m/z = 142.1 (M + H).
4928-87-4
199 suppliers
$9.00/100mg

77-78-1
325 suppliers
$22.00/25g

57031-66-0
77 suppliers
$22.00/250mg
Yield:57031-66-0 85.6%
Reaction Conditions:
with potassium carbonate in acetone at 40; for 4 h;Inert atmosphere;
Steps:
5 Example 5
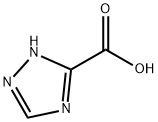
Under the protection of nitrogen, 1,2,4-triazole-3-carboxylic acid (28.3g, 0.25mol), dimethyl sulfate (78.8g, 0.625mol), potassium carbonate (96.6g, 0.7mol) and acetone Put 200mL into the reaction flask, warm up to 40°C for 4 hours, cool to room temperature, filter, rinse the filter cake with acetone, concentrate the filtrate to no flow, add ethyl acetate to dissolve, wash with water to pH=7-8, organic The phase was concentrated and recrystallized from ethanol to obtain 30.2 g of methyl 1-methyl-1H-1,2,4-triazole-3-carboxylate with a yield of 85.6% and HPLC 99.6%.
References:
CN113321622,2021,A Location in patent:Paragraph 0041-0043

4928-88-5
379 suppliers
$5.00/5g

74-88-4
356 suppliers
$15.00/10g

57031-66-0
77 suppliers
$22.00/250mg

4928-87-4
199 suppliers
$9.00/100mg

616-38-6
719 suppliers
$5.00/5G

57031-66-0
77 suppliers
$22.00/250mg