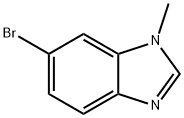
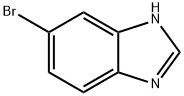
6-BROMO-1-METHYL-1H-BENZO[D]IMIDAZOLE synthesis
- Product Name:6-BROMO-1-METHYL-1H-BENZO[D]IMIDAZOLE
- CAS Number:53484-16-5
- Molecular formula:C8H7BrN2
- Molecular Weight:211.06
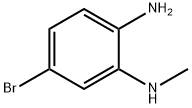
337915-79-4
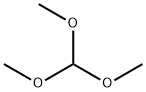
149-73-5
![6-BROMO-1-METHYL-1H-BENZO[D]IMIDAZOLE](/CAS/GIF/53484-16-5.gif)
53484-16-5
General procedure for the synthesis of 6-bromo-1-methyl-1H-benzo[d]imidazole from 4-bromo-2-methylaminoaniline and trimethyl orthoformate: Stannous chloride dihydrate (9.8 g, 43 mmol) was added batchwise to a solution of intermediate 2 (5 g, 21.6 mmol) in ethanol (200 ml), and the mixture was heated and refluxed for 4 h, then concentrated under reduced pressure. The residue was treated with water (200 ml) and 1N sodium hydroxide solution (100 ml). After extraction with dichloromethane, the organic phase was dried with anhydrous sodium sulfate and concentrated under reduced pressure. The residue was dissolved in toluene (50 ml), trimethyl orthoformate (2.6 ml, 24 mmol) and p-toluenesulfonic acid (0.2 g) were added, and the mixture was heated to reflux for 2 h and then concentrated under reduced pressure. The residue was purified by silica gel column chromatography, eluting with dichloromethane/methanol (95/5). 6-Bromo-1-methyl-1H-benzo[d]imidazole was obtained as a cream-colored powder (2.5 g, 54.74%); melting point 126-128 °C.

64-18-6
1132 suppliers
$22.12/250ML
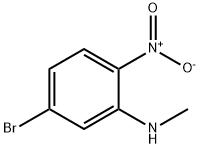
302800-13-1
83 suppliers
$13.00/250mg
![6-BROMO-1-METHYL-1H-BENZO[D]IMIDAZOLE](/CAS/GIF/53484-16-5.gif)
53484-16-5
82 suppliers
$35.00/250mg
Yield: 98%
Reaction Conditions:
with iron;ammonium chloride in isopropyl alcohol for 24 h;Reflux;
Steps:
45.B Step B: Preparation of 6-bromo-1-methyl-benzimidazole
[0444j Step B: Preparation of 6-bromo-1-methyl-benzimidazole: A mixture of 5- bromo-N-methyl-2-nitro-aniline (6.0 g, 26 mmol), iron powder (14.5 g, 260 mmol), ammonium chloride (13.9 g, 260 mmol) and formic acid (49.0 mL, 1298 mmol) in 2- propanol (75 mL) was stirred under reflux for 24 hours. After cooling to ambient temperature, ethyl acetate (50 mL) was added. The solid was removed by filtering through a pad of celite. The filtrate was concentrated. Saturated sodium bicarbonate (20 mL) and ethyl acetate (50 mL) were added. The organic layer was separated, washed with brine, dried (sodium sulfate), filtered and concentrated. The residue was purified by flash chromatography on silica gel 10:1 ethyl acetate/MeOH to give 6-bromo-1-methyl-benzimidazole (5.35 g, 98%) as solid.
References:
PELOTON THERAPEUTICS, INC.;DIXON, Darryl, David;GRINA, Jonas;JOSEY, John, A.;RIZZI, James, P.;SCHLACHTER, Stephen, T.;WALLACE, Eli, M.;WANG, Bin;WEHN, Paul;YANG, Hanbiao WO2015/175845, 2015, A1 Location in patent:Paragraph 0444

337915-79-4
96 suppliers
$39.00/250 mg

149-73-5
548 suppliers
$12.00/5g
![6-BROMO-1-METHYL-1H-BENZO[D]IMIDAZOLE](/CAS/GIF/53484-16-5.gif)
53484-16-5
82 suppliers
$35.00/250mg

302800-13-1
83 suppliers
$13.00/250mg

149-73-5
548 suppliers
$12.00/5g
![6-BROMO-1-METHYL-1H-BENZO[D]IMIDAZOLE](/CAS/GIF/53484-16-5.gif)
53484-16-5
82 suppliers
$35.00/250mg
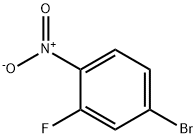
321-23-3
256 suppliers
$10.00/1g
![6-BROMO-1-METHYL-1H-BENZO[D]IMIDAZOLE](/CAS/GIF/53484-16-5.gif)
53484-16-5
82 suppliers
$35.00/250mg
4887-88-1
200 suppliers
$5.00/100mg

74-88-4
356 suppliers
$15.00/10g
![5-Bromo-1-methyl-1H-benzo[d]imidazole](/CAS/GIF/53484-15-4.gif)
53484-15-4
87 suppliers
$25.00/100mg
![6-BROMO-1-METHYL-1H-BENZO[D]IMIDAZOLE](/CAS/GIF/53484-16-5.gif)
53484-16-5
82 suppliers
$35.00/250mg