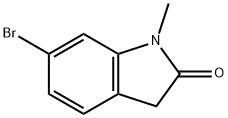
6-Bromo-1-methylindolin-2-one synthesis
- Product Name:6-Bromo-1-methylindolin-2-one
- CAS Number:897957-06-1
- Molecular formula:C9H8BrNO
- Molecular Weight:226.07
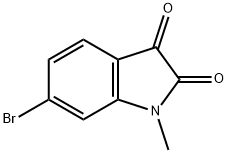
667463-64-1
23 suppliers
$58.00/250mg

897957-06-1
49 suppliers
$49.00/100mg
Yield: 65%
Reaction Conditions:
with hydrazine hydrate at 100 - 125; for 2 h;
Steps:
I-3.3.2 Synthesis of Intermediate 1-3.2
1-3.1 (397 mg, 1.65 mmol) and hydrazine hydrate (1 mL, 20.6 mmol) are heated to 100 °C for 1 h and at 125 °C for 1 h. To the cool reaction mixture DCM and water are added and the aqueous layer extracted twice with DCM. The combined organic layers are washed with brine, dried, concentrated and the residue purified via column chromatography (using solvent mixture cyclohexane / EA = 3:1). Yield 65%. m/z 226 [M+H]+, m/z 224 [M+H]-, rt 0.58 min, LC-MS Method b.
References:
BOEHRINGER INGELHEIM INTERNATIONAL GMBH;GRUNDL, Marc;OOST, Thorsten;PAUTSCH, Alexander;PETERS, Stefan;RIETHER, Doris;WIENEN, Wolfgang WO2013/41497, 2013, A1 Location in patent:Page/Page column 36
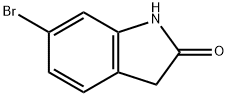
99365-40-9
300 suppliers
$6.00/1g

77-78-1
286 suppliers
$19.69/1ml

897957-06-1
49 suppliers
$49.00/100mg
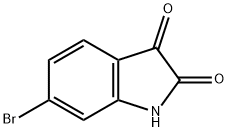
6326-79-0
264 suppliers
$6.00/1g

897957-06-1
49 suppliers
$49.00/100mg