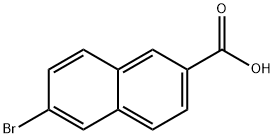
6-Bromo-2-naphthoic acid synthesis
- Product Name:6-Bromo-2-naphthoic acid
- CAS Number:5773-80-8
- Molecular formula:C11H7BrO2
- Molecular Weight:251.08
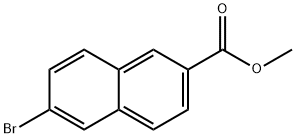
33626-98-1

5773-80-8
Methyl 6-bromo-2-naphthalenecarboxylate (3b) (2.7 g, 10.0 mmol) was used as a raw material to form a suspension with potassium hydroxide (1.1 g, 20.0 mmol) in methanol (50 mL) at 50 °C with vigorous stirring. At the beginning of the reaction, the suspension gradually changed to a homogeneous solution, indicating that the raw material was completely consumed. After the reaction lasted for 8 hours, about 2/3 of the volume of solvent was removed by evaporation under reduced pressure. Subsequently, water (1500 mL) was added to the residue and the unreacted esters were extracted with ethyl acetate. The aqueous phase was acidified to pH 3 with 10% H2SO4 and subsequently extracted with ethyl acetate (3 x 200 mL). The organic phases were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give pure 6-bromo-2-naphthalenecarboxylic acid (3a) (2.1 g, 8.4 mmol, 84% yield). The melting point of the product was 290-294 °C (decomposition). High resolution mass spectrum (ESI+): m/z [M + 1]+ calculated value (C11H8BrO2) was 252.08591, measured value was 252.08582. 1H NMR (DMSO-d6) δ= 7.72 (1H, dd, J = 8.5 and 1.8 Hz, 7-naphthalenyl H), 7.99 (1H, d, J = 8.6 Hz, 4-naphthalenyl H) ), 8.02 (1H, dd, J = 8.6 and 1.4 Hz, 8-naphthalenyl H), 8.09 (1H, d, J = 8.8 Hz, 3-naphthalenyl H), 8.30 (1H, d, J = 1.6 Hz, 5-naphthalenyl H), 8.62 (1H, s, 1-naphthalenyl H), and 13.15 (1H, br.s, COOH).13C NMR ( 125.76 MHz, DMSO-d6) δ= 121.93, 126.50, 127.61, 128.81, 129.83, 130.01, 130.64, 130.90, 131.63, 136.11, 167.30.
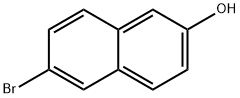
15231-91-1
405 suppliers
$7.00/5g

64-19-7
1628 suppliers
$10.00/25ML

5773-80-8
306 suppliers
$10.00/1g
Yield:5773-80-8 96%
Reaction Conditions:
for 1.5 h;Large scale;
Steps:
6
6-bromo-2-naphthol crystals. Finally, the crude crystals were reintroduced to a mass fraction of 66.7% with a mass of 2160 Kg Of the acetic acid solution in the re-dissolved, dissolved and then static 1. 5h, filtration, washing, After drying, a recrystallization of 6-bromo-2-naphthoic acid was obtained in a yield of 95. 3% pure Degrees 99. 55%, single miscellaneous 0. 45%
References:
Ningbo Jiasi Chemical CO.,LTD,;Zhou, Ming He;Huang, Xi xue CN106542971, 2017, A Location in patent:Paragraph 0035

33626-98-1
322 suppliers
$16.80/1g

5773-80-8
306 suppliers
$10.00/1g
1590-25-6
77 suppliers
$10.00/100mg

5773-80-8
306 suppliers
$10.00/1g
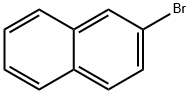
580-13-2
506 suppliers
$6.00/1g

1590-25-6
77 suppliers
$10.00/100mg

75-36-5
588 suppliers
$17.92/100G

5773-80-8
306 suppliers
$10.00/1g

124-38-9
134 suppliers
$175.00/23402
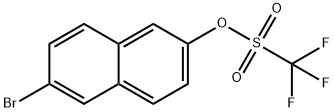
151600-02-1
58 suppliers
$23.00/250mg

5773-80-8
306 suppliers
$10.00/1g