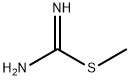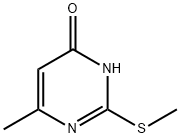
6-Methyl-2-(methylthio)pyrimidin-4-ol synthesis
- Product Name:6-Methyl-2-(methylthio)pyrimidin-4-ol
- CAS Number:6328-58-1
- Molecular formula:C6H8N2OS
- Molecular Weight:156.21
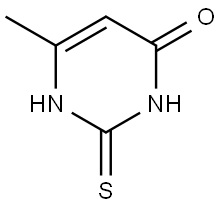
56-04-2

74-88-4

6328-58-1
Sodium hydroxide (97%, 15.7 g, 381 mmol) was added to 500 mL of water and the suspension was stirred for 10 min at room temperature. 6-Methyl-2-thioxasulfinyl-1,2,3,4-tetrahydropyrimidin-4-one (98%, 53.5 g, 369 mmol) was added and the mixture was stirred until completely dissolved for 10 minutes. Iodomethane (28.99 mL, 461 mmol) was slowly added dropwise and the reaction mixture continued to be stirred for 4 hours at room temperature. After completion of the reaction, a colorless solid was obtained by filtration, washed with ice-cold water (2 x 100 mL) and dried under vacuum at 60 °C to give 6-methyl-4-hydroxy-2-methylthiopyrimidine (57 g, 98% yield) as a colorless solid. The product was analyzed by LC-MS: m/z = 156.9 [M + H]+; retention time (RT) = 0.61 min.

56-04-2
381 suppliers
$5.00/100mg

74-88-4
357 suppliers
$15.00/10g

6328-58-1
98 suppliers
$10.60/1gm:
Yield: 98%
Reaction Conditions:
with sodium hydroxide in water at 20; for 4 h;
Steps:
1.1.1 Step 1: Synthesis of 6-methyl-2-(methylsulfanyl)-3,4-dihydropyrimidin-4-one
(EV-AO5743-001) [00128] To water (500 mL) was added NaOH (97%, 15.7 g, 381 mmol) and the suspension stirred at r.t. for 10 mins. 6-methyl-2-sulfanylidene-l,2,3,4-tetrahydropyrimidin-4-one (98%, 53.5 g, 369mmol) was added and the mixture stirred until fully dissolved for 10 mins. lodomethane (28.99 mL, 461 mmol) was added dropwise and the mixture stirred at r.t. for 4 h. The colorless solid was filtered, washed with ice cold water (2 x 100 mL) and dried under vacuum at 60 °C to afford the title compound (57 g, 98%) as a colorless solid. [00129] Method A: LC-MS m/z = 156.9 [M + H]+; RT = 0.61 min.
References:
QUARTET MEDICINE, INC.;ECOLE POLYTECHNIQUE FEDERALE DE LAUSANNE (EPFL);TEBBE, Mark, Joseph;ATTON, Holly, Victoria;AVERY, Craig;BROMIDGE, Steven, Mark;KERRY, Mark;KOTEY, Adrian, Kotei;MONCK, Nathaniel, J.;MENICONI, Mirco;RIDGILL, Mark, Peter;TYE, Heather;SAIAH, Eddine;JOHNSSON, Kai, Peter;GORSKA, Katarzyna, Irena;PENG, Hairuo;MCCALL, John, Michael WO2017/59191, 2017, A1 Location in patent:Paragraph 00126-00129

56-04-2
381 suppliers
$5.00/100mg

74-88-4
357 suppliers
$15.00/10g

6328-58-1
98 suppliers
$10.60/1gm:

141-97-9
825 suppliers
$5.00/25g
867-44-7
378 suppliers
$5.00/10g

6328-58-1
98 suppliers
$10.60/1gm:

56-04-2
381 suppliers
$5.00/100mg

77-78-1
319 suppliers
$19.69/1ml

6328-58-1
98 suppliers
$10.60/1gm: