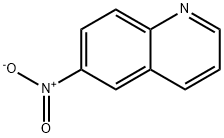
6-NITROQUINOLINE synthesis
- Product Name:6-NITROQUINOLINE
- CAS Number:613-50-3
- Molecular formula:C9H6N2O2
- Molecular Weight:174.16
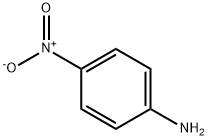
5332-25-2

613-50-3
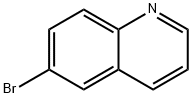
GENERAL STEPS: 6-bromoquinoline (0.5 mmol), copper(II) trifluoromethanesulfonate (45 mg, 0.125 mmol), potassium nitrite (KNO2, 128 mg, 1.5 mmol), and anhydrous dimethylsulfoxide (DMSO, 0.6 mL) were sequentially added to a pre-dried oven-dried pressure tube under nitrogen protection. The pressure tube was sealed with a PTFE screw cap with a micro-valve and purged by nitrogen for 5 min to remove air. Subsequently, the reaction mixture was stirred at room temperature for 10 minutes and then gradually warmed up to 130°C and the reaction was continued at this temperature for 48 hours. Upon completion of the reaction, the mixture was cooled to room temperature, washed with excess ice water and extracted with ethyl acetate (3 x 10 mL). The organic phases were combined, dried over anhydrous sodium sulfate (Na2SO4), filtered and concentrated under reduced pressure to give the crude product. The crude product was purified by column chromatography on silica gel (for entries 1-18 in Table 2) or basic alumina (for entries 19-23 in Table 2), using a solvent mixture of ethyl acetate and hexane as eluent, and the target product, 6-nitroquinoline, was finally obtained in good yield.
612-57-7
213 suppliers
$6.00/1g

613-50-3
235 suppliers
$10.00/5g
Yield:613-50-3 97%
Reaction Conditions:
with Tris(3,6-dioxaheptyl)amine;sodium nitrite;tris-(dibenzylideneacetone)dipalladium(0);t-BuBrettPhos in tert-butyl alcohol at 130; for 24 h;Product distribution / selectivity;Inert atmosphere;
Steps:
54
EXAMPLE FIFTY-FOUR: General Procdure for Pd-Catalyzed Nitrations of Aryl Chlorides and Aryl Sulfonates (Figures 20 and 21); An oven-dried schlenk tube, which was equipped with a magnetic stir bar and fitted with a rubber septum, was charged with the Pd2(dba)3 (0.5 mol %), ligand (6, 25, 26, or 27) (1.2 mol %), and NaNO2 (138 mg, 2.0 mmol) (aryl halides* that were solids at room
References:
MASSACHUSETTS INSTITUTE OF TECHNOLOGY WO2009/76622, 2009, A2 Location in patent:Page/Page column 144-145
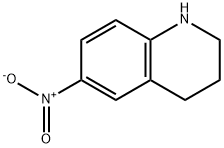
14026-45-0
87 suppliers
inquiry

613-50-3
235 suppliers
$10.00/5g

5332-25-2
396 suppliers
$6.00/5g

613-50-3
235 suppliers
$10.00/5g
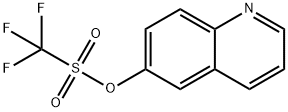
173089-80-0
29 suppliers
$80.40/5g

613-50-3
235 suppliers
$10.00/5g