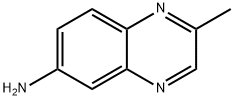
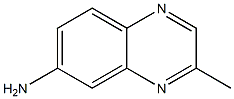
6-Quinoxalinamine,2-methyl-(9CI) synthesis
- Product Name:6-Quinoxalinamine,2-methyl-(9CI)
- CAS Number:4188-17-4
- Molecular formula:C9H9N3
- Molecular Weight:159.19
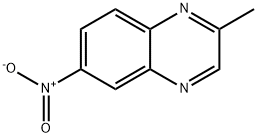
2942-02-1
12 suppliers
inquiry

4188-17-4
7 suppliers
inquiry
Yield:4188-17-4 89%
Reaction Conditions:
with hydrogen;palladium 10% on activated carbon in methanol; under 760.051 Torr; for 4 h;
Steps:
Synthesis of 3-chloro-N- (2-methvl-quinoxalin-6-yl)-2-methvl- benzenesulfonamide, STX 955 (KRB01079):; 6-amino-2-methylquinoxaline (KRB01078): To a solution of 2-methyl-6-nitroquinoxaline [23] (500 mg, 2.64 mmol) in methanol (22 mL) was added 10% palladium on carbon (50 mg) and the mixture was stirred under 1 atm H2 for 4 h. The mixture was filtered through celite and the filtrate evaporated. The residue was passed through a silica plug and evaporated to afford 6-amino-2-methylquinoxaline as a yellow solid (376 mg, 89%), single spot at Rf 0.33 (ethyl acetate). mp 163-164°C ([23] 164-165°C). 1H NMR (CDCI3) : 5 8.57 (1H, s), 7.78 (1H, d, J=8.4 Hz), 7.17-7. 12 (2H, m), 4.11 (2H, s-NH2), 2.67 (3H, s).
References:
WO2005/42513,2005,A1 Location in patent:Page/Page column 69
7705-07-9
118 suppliers
$75.70/250ML
497-19-8
1488 suppliers
$14.00/1KG
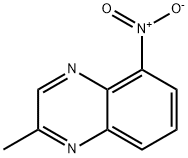
76982-28-0
9 suppliers
inquiry

4188-17-4
7 suppliers
inquiry
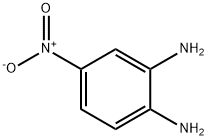
99-56-9
401 suppliers
$6.00/5g

4188-17-4
7 suppliers
inquiry
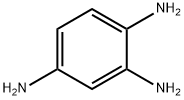
615-71-4
20 suppliers
inquiry
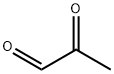
78-98-8
307 suppliers
$10.60/1gm:
4236-41-3
2 suppliers
inquiry

4188-17-4
7 suppliers
inquiry
