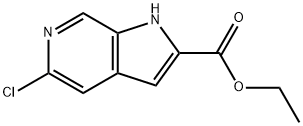
ethyl 5-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxylate synthesis
- Product Name:ethyl 5-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxylate
- CAS Number:800401-67-6
- Molecular formula:C10H9ClN2O2
- Molecular Weight:224.64
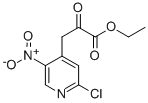
800401-66-5
![ethyl 5-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxylate](/CAS/GIF/800401-67-6.gif)
800401-67-6
Step 2: Synthesis of ethyl 5-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxylate 10-b To a solution of 3-(2-chloro-5-nitropyridin-4-yl)-2-oxopropanoic acid ethyl ester 10-a (2.726 g, 10 mmol) in THF (80 mL) and EtOH (30 mL) was added a saturated ammonium chloride solution (50 mL). Subsequently, iron powder (2.747 g, 4.9 eq.) was added in batches at room temperature under vigorous stirring, and then the mixture was heated to reflux for 2 hours. Upon completion of the reaction, the mixture was cooled to room temperature, filtered through diatomaceous earth and washed with warm THF/ethanol (1:1, v/v). The filtrate was evaporated and the residue was dissolved in 100 mL of water with stirring and refluxing. The resulting precipitate was thermally filtered, washed twice with warm water and then dried under vacuum to give ethyl 5-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxylate 10-b (1.9 g, 84% yield). m/z = 225 (M + H)+; 1H NMR (400 MHz, DMSO-d6) δ ppm: 1.36 (t, J = 6.8 Hz, 3H), 4.39 (q, J = 6.7 Hz, 2H), 7.15 (s, 1H), 7.76 (s, 1H), 8.64 (s, 1H), 12.59 (br s, 1H).

800401-66-5
20 suppliers
inquiry
![ethyl 5-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxylate](/CAS/GIF/800401-67-6.gif)
800401-67-6
103 suppliers
$98.00/50mg
Yield:800401-67-6 84%
Reaction Conditions:
with iron;ammonium chloride in tetrahydrofuran;ethanol at 20; for 2 h;Reflux;
Steps:
1a.2
Step 2: synthesis of ethyl 5-chloro-lH-pyrrolo[2,3-c]pyridine-2-carboxylate 10-bTo a solution of ethyl 3-(2-chloro-5-nitropyridin-4-yl)-2-oxopropanoate 10-a (2.726 g, 10 mmoles) in THF (80 mL) and EtOH (30 mL) was added a saturated solution of ammonium chloride (50 mL). Then, iron (2.747 g, 4.9 eq) was added portionwise under vigorous stirring to the mixture at room temperature, which was subsequently heated at reflux for 2h. The mixture was cooled down to RT, filtered over dicalite and washed with warm THF/ethanol 1/1. The filtrate was evaporated and the residue was stirred and refluxed in 100 mL water. The resulting precipitate was filtered off hot, washed twice with warm water and then dried in vacuo to provide 1.9 g (84% yield) of the targeted compound 10-b. m/z = 225 (M+H)+; 1H MR (400 MHz, OMSO-d6) ppm 1.36 (t, J = 6.8 Hz, 3H), 4.39 (q, J = 6.7 Hz, 2H), 7.15 (s, lH), 7.76 (s, lH), 8.64 (s, lH), 12.59 (br s,lH).
References:
JANSSEN R&D IRELAND;COOYMANS, Ludwig Paul;DEMIN, Samuël Dominique;HU, Lili;JONCKERS, Tim Hugo Maria;RABOISSON, Pierre Jean-Marie Bernard;TAHRI, Abdellah;VENDEVILLE, Sandrine Marie Helene WO2012/80450, 2012, A1 Location in patent:Page/Page column 24
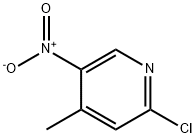
23056-33-9
346 suppliers
$3.00/1g
![ethyl 5-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxylate](/CAS/GIF/800401-67-6.gif)
800401-67-6
103 suppliers
$98.00/50mg
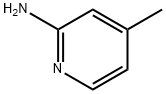
695-34-1
537 suppliers
$5.00/10g
![ethyl 5-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxylate](/CAS/GIF/800401-67-6.gif)
800401-67-6
103 suppliers
$98.00/50mg
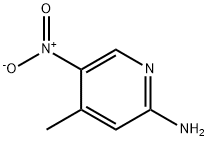
21901-40-6
300 suppliers
$6.00/1g
![ethyl 5-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxylate](/CAS/GIF/800401-67-6.gif)
800401-67-6
103 suppliers
$98.00/50mg