
ethyl 4-cyano-1-{[2-(trimethylsilyl)ethoxy]methyl}-1H-imidazole-2-carboxylate synthesis
- Product Name:ethyl 4-cyano-1-{[2-(trimethylsilyl)ethoxy]methyl}-1H-imidazole-2-carboxylate
- CAS Number:854044-52-3
- Molecular formula:C13H21N3O3Si
- Molecular Weight:295.41
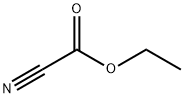
623-49-4
243 suppliers
$10.60/1gm:
![ethyl 4-cyano-1-{[2-(trimethylsilyl)ethoxy]methyl}-1H-imidazole-2-carboxylate](/CAS/GIF/854044-52-3.gif)
854044-52-3
12 suppliers
inquiry
Yield:854044-52-3 74%
Reaction Conditions:
Stage #1: 2-bromo-1-{[2-(trimethylsilyl)ethoxy]methyl}-1H-imidazole-4-carbonitrilewith isopropylmagnesium chloride in tetrahydrofuran at -40; for 0.166667 h;
Stage #2: ethyl cyanoformate in tetrahydrofuran at 20; for 1 h;Product distribution / selectivity;
Steps:
3.c
To a solution of 2-bromo-l-(2-trimethylsilanyl-ethoxymethyl)-lH-imidazole-4- carbonitrile (0.55 g, 1.8 mmol) (as prepared in the previous step) in TΗF (6 mL) at -40 0C was added drop wise a solution of 2M i-PrMgCl in TΗF (1 mL). The reaction was allowed to stir for 10 min at -400C and then cooled to -78 0C, and ethyl cyanoformate (0.3 g, 3.0 mmol) was added. The reaction allowed to attain RT and stirred for 1 h. The reaction was quenched with satd aq NH4Cl, diluted with EtOAc (20 mL) and washed with brine (2 x 20 EPO
References:
WO2006/47277,2006,A2 Location in patent:Page/Page column 42-43
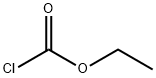
541-41-3
0 suppliers
$21.00/5g
![ethyl 4-cyano-1-{[2-(trimethylsilyl)ethoxy]methyl}-1H-imidazole-2-carboxylate](/CAS/GIF/854044-52-3.gif)
854044-52-3
12 suppliers
inquiry
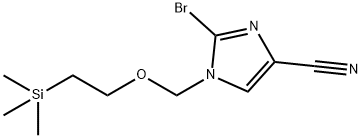
854044-51-2
37 suppliers
inquiry

64-17-5
764 suppliers
$10.00/50g
201230-82-2
1 suppliers
inquiry
![ethyl 4-cyano-1-{[2-(trimethylsilyl)ethoxy]methyl}-1H-imidazole-2-carboxylate](/CAS/GIF/854044-52-3.gif)
854044-52-3
12 suppliers
inquiry

854044-51-2
37 suppliers
inquiry

623-49-4
243 suppliers
$10.60/1gm:
![ethyl 4-cyano-1-{[2-(trimethylsilyl)ethoxy]methyl}-1H-imidazole-2-carboxylate](/CAS/GIF/854044-52-3.gif)
854044-52-3
12 suppliers
inquiry