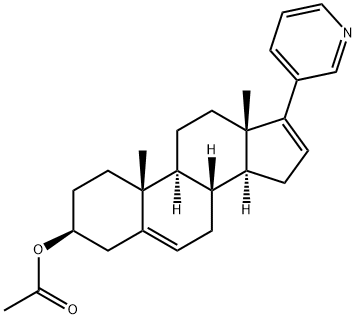
Abiraterone acetate synthesis
- Product Name:Abiraterone acetate
- CAS Number:154229-18-2
- Molecular formula:C26H33NO2
- Molecular Weight:391.55

Madhra, Mukesh Kumar; Sriram, Hari Mohan; Inamdar, Murad; Sharma, Mukesh Kumar; Prasad, Mohan; Joseph, Sony. Improved Procedure for Preparation of Abiraterone Acetate. Organic Process Research & Development. Volume 18. Issue 4. Pages 555-558. Journal; Online Computer File. (2014).

108-24-7
0 suppliers
$14.00/250ML
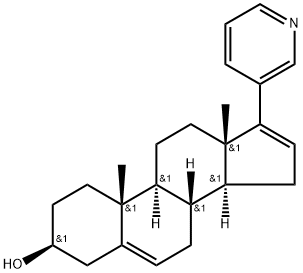
154229-19-3
413 suppliers
$28.00/1mg

154229-18-2
563 suppliers
$28.00/1unit
Yield:154229-18-2 96.7%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 3 h;
Steps:
2; 3; 4
6.4 g (0.018 mol) of abiraterone, 50 mL of dichloromethane, and 3.0 g (0.03 mol) of triethylamine were added to the reaction flask. The temperature was lowered to 0-5°C, and 3.0 g (0.03 mol) of acetic anhydride was slowly added dropwise. After dropping, the temperature was raised to room temperature, and maintain temperature reaction for 3 hours. Filter and wash the organic layer once with 20 mL of water, 20 mL of 1 N aqueous sodium bicarbonate solution. The organic layer was concentrated to dryness under reduced pressure, 20 mL of 90% ethanol aqueous solution was added to the concentrate, and the temperature was raised to reflux to make the solution clear. The temperature was lowered to 0-5° C. for two hours, crystallized, filtered, and dried in vacuo to obtain 6.8 g of crude abiraterone acetate with a molar yield of 96.7% and HPLC purity of 98.3%. The crude product was recrystallized with acetone to obtain 6.2 g of abiraterone acetate with a purity of 99.6%.
References:
CN114853838,2022,A Location in patent:Paragraph 0092-0093; 0096; 0097-0098; 0101; 0102-0103; 0106
115375-60-5
56 suppliers
inquiry
89878-14-8
400 suppliers
$6.00/1g

154229-18-2
563 suppliers
$28.00/1unit

75-36-5
589 suppliers
$17.92/100G

154229-19-3
413 suppliers
$28.00/1mg

154229-18-2
563 suppliers
$28.00/1unit

115375-60-5
56 suppliers
inquiry
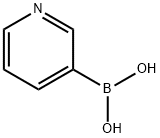
1692-25-7
486 suppliers
$5.00/1g

154229-18-2
563 suppliers
$28.00/1unit
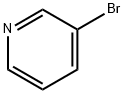
626-55-1
573 suppliers
$5.00/5/ G

25256-95-5
0 suppliers
inquiry

154229-18-2
563 suppliers
$28.00/1unit





