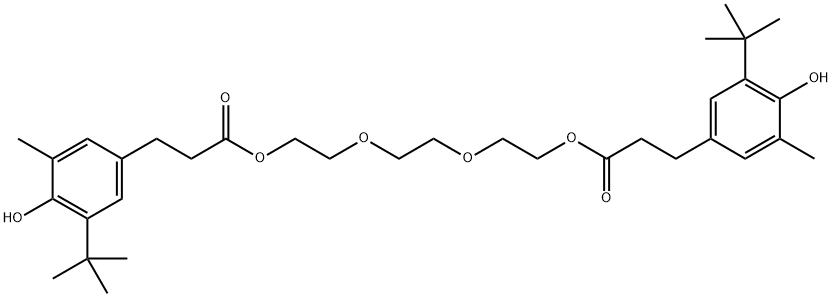
Antioxidant 245 synthesis
- Product Name:Antioxidant 245
- CAS Number:36443-68-2
- Molecular formula:C34H50O8
- Molecular Weight:586.77
6386-39-6

51590-67-1
856335-90-5

36443-68-2
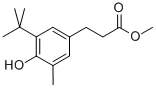
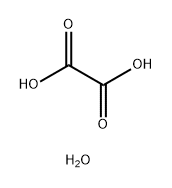
(2) To a 1 L four-necked flask equipped with a stirrer, a condenser tube, a thermometer and a nitrogen introduction tube were added 327.9 g (1.31 mol) of methyl 3-(3-tert-butyl-4-hydroxy-5-methylphenyl)propionate, 85.6 g (0.57 mol) of triethylene glycol and 0.9 g (0.00431 mol) of monobutyltin oxide. The mixture was reacted at 170 °C for 1 h while the resulting methanol was distilled. Subsequently, the reaction was continued for 8 h at a reduced pressure of 60-70 mmHg and nitrogen was continuously vented into the solution to ensure complete reaction. Upon completion of the reaction, 40.5 g of excess methyl 3-(3-tert-butyl-4-hydroxy-5-methylphenyl)propionate was distilled by film distillation at 150-185 °C and 0.5-0.2 mmHg. This distillate can be used directly in the subsequent reaction. The reaction mixture was cooled to 115 °C and restored to atmospheric pressure by passing nitrogen, followed by addition of 260 g of toluene to dissolve the mixture homogeneously. The resulting solution was washed once with 200 g (0.11 mol) of 5% aqueous oxalic acid at 80 °C and twice with 200 g of water to separate the organic and aqueous layers. The organic layer was concentrated at 120 °C and 30 mmHg to give a pale yellow viscous substance. The mucilage was recrystallized with a mixed solvent of methanol-water to obtain 317.8 g of white crystalline triethylene glycol bis[3-(3-tert-butyl-4-hydroxy-5-methylphenyl)propionate]. The yield was 95% and the residual tin content was 2 ppm.

6386-39-6
18 suppliers
inquiry

51590-67-1
132 suppliers
inquiry

856335-90-5
0 suppliers
inquiry

36443-68-2
255 suppliers
$5.00/250mg
Yield:36443-68-2 95%
Reaction Conditions:
with nitrogen in water;toluene;2,2'-[1,2-ethanediylbis(oxy)]bisethanol;
Steps:
2.2 EXAMPLE 2
(2) Into a 1-liter four-necked flask equipped with a stirrer, a condenser, a thermometer, and a nitrogen introducing tube were introduced 327.9 g (1.31 moles) of methyl 3-(3-tert-butyl-4-hydroxy-5-methylphenyl) propionate, 85.6 g (0.57 mole) of triethylene glycol, and 0.9 g (0.00431 mole) of monobutyltin oxide. The mixture was allowed to react at 170° C. for 1 hour, and the resulting methanol was distilled off. The reaction was continued for further 8 hours under a reduced pressure of 60-70 mmHg while bubbling nitrogen gas into the solution and thus completed. Excess methyl 3-(3-tert-butyl-4-hydroxy-5-methylphenyl) propionate was distilled off in an amount of 40.5 g at 150°-185° C. under 0.5 -0.2 mmHg by thin film distillation. This distillate can be reused, as it is, for the next reaction. Then, the reaction mixture was cooled to 115° C. and returned to atmospheric pressure by the introduction of nitrogen gas, and dissolved homogeneously by adding 260 g of toluene. The solution was washed with 200 g (0.11 mole) of 5% oxalic acid water at 80° C. and then with 200 g of water twice for partitioning to two layers. The toluene layer was concentrated at 120° C. under 30 mmHg to give a pale yellow tacky substance. The pale yellow tacky substance so obtained was recrystallized from methanol-water to Yield 317.8 g of white crystals of triethylene glycol bis[3-(3-tert-butyl-4-hydroxy-5-methylphenyl)propionate] as intended. Yield: 95%, residual tin content: 2 ppm.
References:
US5081280,1992,A