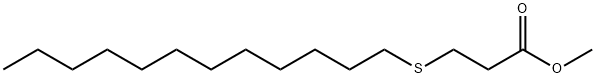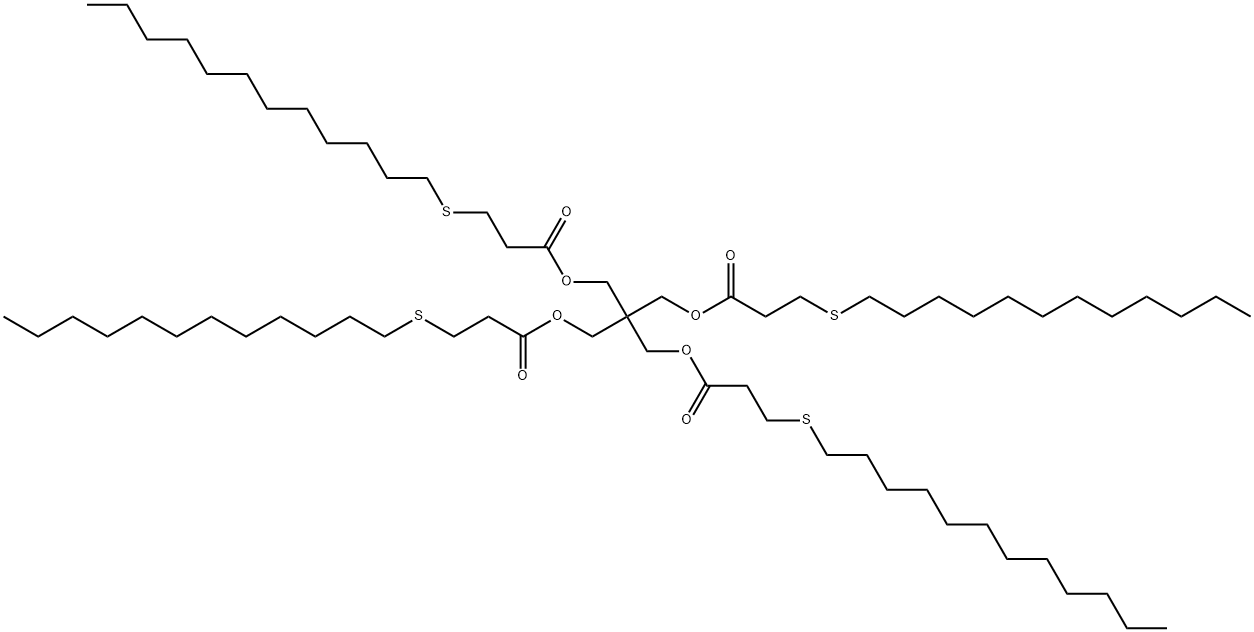
Antioxidant 412S synthesis
- Product Name:Antioxidant 412S
- CAS Number:29598-76-3
- Molecular formula:C65H124O8S4
- Molecular Weight:1161.94
Yield:29598-76-3 98.2%
Reaction Conditions:
with [BCl2(1-methylimidazole)][Al2Cl7] at 120; for 3 h;Large scale;Reagent/catalyst;Temperature;
Steps:
1.2; 1.3; 2.2; 2.3; 3.2; 3.3 (2) Preparation of antioxidant 412S:
Import the n-dodecyl thiopropionate methyl ester obtained in step (1) into the transesterification reactor with a pump, add 6Kg of pentaerythritol and 200g [BCl2(mim)][Al2Cl7], turn on the stirring and raise the temperature to about 120. The transesterification reaction is carried out for 3 hours, and distillation is reduced at this temperature to remove toluene and methanol generated by the reaction to complete the reaction. In the vacuum distillation process, the waste gas is condensed to produce non-condensable gas (G2). After the reaction, the transesterification reaction liquid was pumped into a dissolving kettle pre-added with 20L of acetone, activated carbon was added, and stirred for 30 minutes. Then, the transesterification reaction solution dissolved in acetone is pressure-filtered through a filter to filter out the waste activated carbon (Sl). After pressing the material, the reaction liquid is transferred to the crystallization kettle, the stirring is turned on, the temperature is raised to reflux and kept for 30 minutes, and then the cooling and crystallization are started. After the crystallization is completed, the crystallization liquid is centrifuged to obtain a wet product. The mother liquor enters the mother liquor tank; the centrifuge for crystallization separation is a fully enclosed automatic bottom-discharge centrifuge. The crystallization mother liquor and the centrifuge drum rotate at high speed during the centrifugation process The generated gas flow enters the gas-liquid separator, and after gas-liquid separation, the crystallization mother liquor enters the crystallization mother liquor intermediate balance tank; the gas flow passes through the gas phase balance pipe connected to the centrifuge and returns to the centrifuge to form a closed gas phase cycle and produce a small amount of unbalanced gas (G3). The wet filter cake is transported by a fully enclosed screw auger to the wet product silo for temporary storage, and then added to the vacuum rake dryer, and vacuum dried under controlled temperature. After the vacuum drying exhaust gas is condensed to recover the solvent, a small amount of non-condensable gas (G4) is generated. After the drying is completed, the powdered product is delivered to the ten-product silo, and then sieved through a vibrating screen, and then packed into the white dynamic packaging machine to obtain the finished product of antioxidant 412S. (3) Mother liquor recovery process: The crystallization mother liquor after centrifugal separation is carried into the crystallization mother liquor intermediate tank, and is pumped to the crystallization mother liquor rectification tower to continuously rectify and recover the crystallization solvent. Distillation waste gas (exhaust gas G5) is generated during the distillation process, and the recovered acetone is recycled and reused. The obtained mother liquor rectification concentrate is mainly light brown antioxidant, after being recrystallized, it is crystallized and combined with the antioxidant 412S pentaerythritol tetrakis (3-lauryl thiopropionate) obtained in step (2) above. The antioxidant 412S pentaerythritol tetrakis (3-lauryl thiopropionate) 28.1Kg was obtained, the reaction yield was 98.2%, and the purity was 99.8%.
References:
CN112300039,2021,A Location in patent:Paragraph 0029; 0032-0039; 0042-0049; 0052-0058
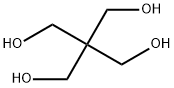
115-77-5
6 suppliers
$10.00/10g

112-41-4
217 suppliers
$8.00/10g
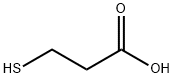
107-96-0
513 suppliers
$6.00/25g

29598-76-3
85 suppliers
$25.00/25g

115-77-5
6 suppliers
$10.00/10g
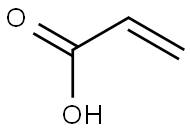
79-10-7
681 suppliers
$20.50/500ml
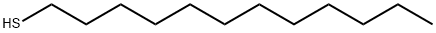
112-55-0
317 suppliers
$14.00/5mL

29598-76-3
85 suppliers
$25.00/25g