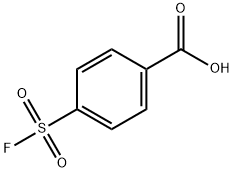
Benzoic acid, 4-(fluorosulfonyl)-, ethyl ester synthesis
- Product Name:Benzoic acid, 4-(fluorosulfonyl)-, ethyl ester
- CAS Number:366-85-8
- Molecular formula:C9H9FO4S
- Molecular Weight:232.23
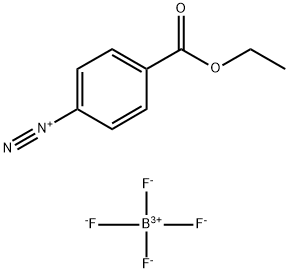
348-06-1
3 suppliers
inquiry

366-85-8
4 suppliers
inquiry
Yield:366-85-8 58%
Reaction Conditions:
with sodium metabisulfite;N-fluorobis(benzenesulfon)imide in water;acetonitrile at 60; for 6 h;Inert atmosphere;Sandmeyer Reaction;
Steps:
4.3 General procedures for fluorosulfonylation of aryldiazonium tetrafluoroborates
General procedure: To a mixture of arenediazonium tetrafluoroborate (0.2mmol, 1.0 equiv.) and NFSI (94.5mg, 0.3mmol, 1.5 equiv.) in MeCN/H2O (4.0/0.2mL) was added Na2S2O5 (76.0mg, 0.4mmol, 2.0 equiv.). The reaction mixture was stirred at 60°C under nitrogen atmosphere for 6h. After the reaction was complete, brine was added. The resulting mixture was extracted with EtOAc for three times. The combined organic layer was washed with brine, dried over anhydrous Na2SO4, and concentrated under reduced pressure. The resulting residue was purified by silica gel flash column chromatography to give the product.
References:
Huang, Yangen;Liu, Shuai;Qing, Feng-Ling;Xu, Xiu-Hua [Journal of Fluorine Chemistry,2020,vol. 240,art. no. 109653]
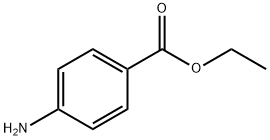
94-09-7
0 suppliers
$5.00/50mg

366-85-8
4 suppliers
inquiry
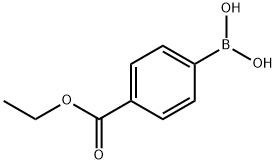
4334-88-7
287 suppliers
$6.00/1g

366-85-8
4 suppliers
inquiry
10130-89-9
178 suppliers
$7.00/250mg

366-85-8
4 suppliers
inquiry