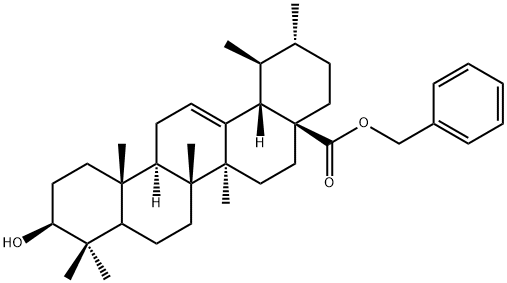
Benzyl ursolate synthesis
- Product Name:Benzyl ursolate
- CAS Number:192211-41-9
- Molecular formula:C37H54O3
- Molecular Weight:546.82
77-52-1
637 suppliers
$29.00/100mg

100-39-0
428 suppliers
$10.00/10g

192211-41-9
6 suppliers
inquiry
Yield:192211-41-9 97%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 20; for 6 h;Inert atmosphere;
Steps:
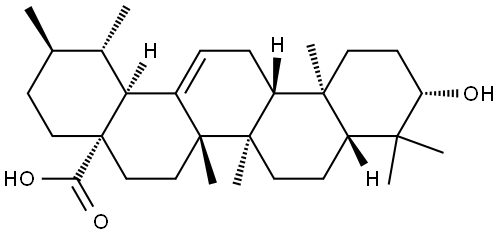
1.1 Synthesis of (lS,2R,4aS,6aS,6bR,8aR,10S,12aR,12bR,14bS)-benzyl 10-hydroxy- 1,2,6a, 6b,9,9,12a-heptamethyl-l,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,l l, 12,12a, 12b,13, 14b-icosa hydropicene-4a-carboxylate:
To a suspension of (lS,2R,4aS,6aS,6bR,8aR,10S,12aR,12bR, 14bS)-10-hydroxy- 1,2,6a, 6b,9,9,12a-heptamethyl-l,2,3,4,4a,5,6,6a,6b,7,8,8a,9,10,l l, 12,12a, 12b,13, 14b-icosa hydropicene-4a-carboxylic acid (50g, 109 mmol) in DMF (500mL) was added potassium carbonate salt (40g, 289mmol) under nitrogen atmosphere followed by addition of bromomethyl benzene (16.90mL, 142mmol) to the mixture. The reaction mixture was stirred for 6h at room temperature and on completion of reaction the reaction mixture was poured into cold water (5L). The separated solid material was collected and washed with acetonitrile (500mL) to afford pure title compound 2 (58g) as white solid. Yield: 97%; JH NMR (300MHz, DMSO-de): δ 0.54 (s, 3H), 0.66 (s, 3H), 0.82 (s, 3H), 0.90 (d, / = 9.0 Hz, 6H), 1.0 (s, 3H), 0.99-1.07 (m, 3H), 1.42-2.15 (m, 23H), 2.98 (bs, 1H), 4.31 (bs, 1H), 4.94 (d, / = 12.0 Hz, 1H), 5.04 (d, / = 12.0 Hz, 1H), 5.13 (s, 1H), 7.32 (bs, 5H); MS(ES+): 547.8 (M+l).
References:
WO2015/198232,2015,A1 Location in patent:Page/Page column 80; 81

77-52-1
637 suppliers
$29.00/100mg

100-44-7
654 suppliers
$13.50/250G

192211-41-9
6 suppliers
inquiry