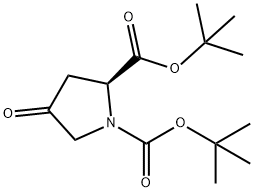
BOC-4-OXO-L-PROLINE TERT-BUTYL ESTER synthesis
- Product Name:BOC-4-OXO-L-PROLINE TERT-BUTYL ESTER
- CAS Number:166410-05-5
- Molecular formula:C14H23NO5
- Molecular Weight:285.34
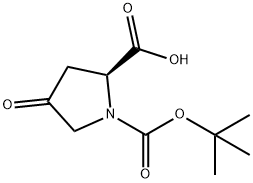
84348-37-8

75-65-0

166410-05-5
For the synthesis of di-tert-butyl (S)-4-oxopyrrolidine-1,2-dicarboxylate (16c), (S)-1-(tert-butoxycarbonyl)-4-oxopyrrolidine-2-carboxylic acid (16b, 1.35 g, 5.9 mmol) was dissolved in anhydrous dichloromethane (27 mL) and the solution was cooled to 0 °C. Subsequently, tert-butanol (1.7 mL, 17.7 mmol) and 4-dimethylaminopyridine (DMAP, 72 mg, 0.59 mmol) were sequentially added to the solution. After stirring for 5 min, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC-HCl, 1.19 g, 6.2 mmol) was added. The reaction mixture was gradually warmed to room temperature and continued to stir overnight. Upon completion of the reaction, the reaction was quenched with saturated sodium bicarbonate solution and extracted three times with dichloromethane. The organic layers were combined, washed with saturated brine, dried over anhydrous magnesium sulfate and filtered. The solvent was removed by concentration under reduced pressure and the resulting residue was purified by fast column chromatography (eluent: n-hexane/ethyl acetate, 8:1) to afford the target product 16c (0.96 g, 57% yield) as a light yellow oil.
170850-75-6
63 suppliers
$75.70/250MG

166410-05-5
154 suppliers
$55.00/1g
Yield: 92.74%
Reaction Conditions:
with 4-methylmorpholine N-oxide in dichloromethane at 20; for 1.5 h;Reagent/catalyst;
Steps:
1.2
(2) 17.36 g of the compound hydrazine was dissolved in dichloromethane, and 9.45 g of N-methyl_N_oxidized morpholine was added and stirred at room temperature for 1.5 hours, and the reaction completely gave a black turbid liquid, and the reaction solution was filtered off to remove insoluble matter. The filtrate was evaporated to dryness and purified by column chromatography to yield 16.75 g of white solid compound m, yield 92.74%
References:
Tierxi (Nanjing) Pharmaceutical Research And Development Co., Ltd.;He Xu;Wang Jiayan;Zhang Chi;Liu Chun;Cui Xilin CN110028496, 2019, A Location in patent:Paragraph 0056; 0059; 0069

84348-37-8
288 suppliers
$5.00/250mg

75-65-0
783 suppliers
$10.00/10ml

166410-05-5
154 suppliers
$55.00/1g
1634-04-4
692 suppliers
$14.00/100g

84348-37-8
288 suppliers
$5.00/250mg

166410-05-5
154 suppliers
$55.00/1g

24424-99-5
869 suppliers
$13.50/25G

166410-05-5
154 suppliers
$55.00/1g
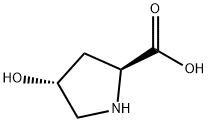
51-35-4
884 suppliers
$6.00/5g

166410-05-5
154 suppliers
$55.00/1g