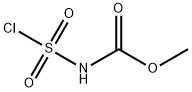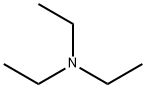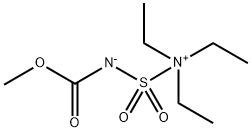
Burgess reagent synthesis
- Product Name:Burgess reagent
- CAS Number:29684-56-8
- Molecular formula:C8H18N2O4S
- Molecular Weight:238.3
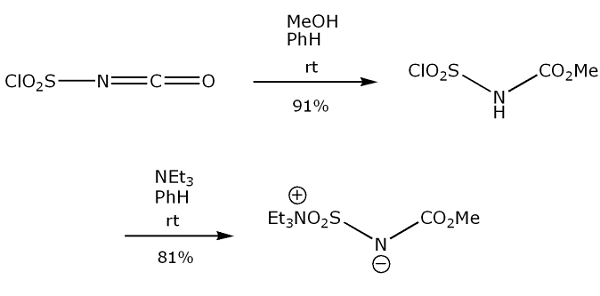
Yield:29684-56-8 63%
Reaction Conditions:
in benzene at 20;
Steps:
Synthesis of Methyl(carboxysulfamoyl)triethylammonium Hydroxide 1
Methyl(carboxysulfamoyl)triethylammonium hydroxide 1 was prepared using the Organic Syntheses procedure published by Burgess et al.[2] Compound 1 was obtained as colourless crystals (mp 67.2-69.5 C, lit[2] mp 71-728C), yield 63 %. dH (600 MHz, CDCl3) 1.41 (9H, t, JHH 7.2, NCH2CH3), 3.47 (6H, q, JHH 7.2, NCH2CH3, 3.70 (3H, s, CH3), dC (151 MHz, CDCl3) 9.38 (NCH2CH3), 50.43 (NCH2CH3), 53.26 (OCH3), 158.2 (C=O). nmax (CHCl3)/cm_1 3020 (w), 2951 (w), 1698 (m), 1460 (w), 1438 (w), 1342 (w), 1262 (s), 1222 (w), 1202 (w), 1187 (w), 1110 (w), 1039 (w), 965 (w), 866 (w), 734 (w), 714 (w), 668 (w), 623 (w), 590 (w), 577 (w), 563 (w). nmax (solid)/cm_1 3046 (w), 2996 (w), 2979 (w), 2951 (w), 1719 (w), 1688 (m), 1455 (m), 1441 (m), 1402 (w), 1338 (w), 1326 (m), 1304 (w), 1241 (s), 1219 (m), 1186 (m), 1111 (m), 1092 (m), 1042 (m), 1023 (m), 960 (m), 893 (w), 856 (m), 835 (m), 787 (m), 717 (m), 695 (w), 679 (w), 668 (w), 651 (w), 641 (w), 627 (m), 600 (s), 577 (m).
References:
Arduengo, Anthony J.;Uchiyama, Yosuke;DIxon, David A.;Vasiliu, Monica [Australian Journal of Chemistry,2019,vol. 72,# 11,p. 867 - 873]