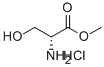
D-Serine methyl ester hydrochloride synthesis
- Product Name:D-Serine methyl ester hydrochloride
- CAS Number:5874-57-7
- Molecular formula:C4H10ClNO3
- Molecular Weight:155.58

67-56-1
312-84-5

5874-57-7
General procedure for the synthesis of D-serine methyl ester hydrochloride from methanol and D-serine: 4.2.1.1 Synthesis of methyl (R)-2-isopropyl-4,5-dihydrooxazole-4-carboxylate First, sulfoxide chloride (SOCl?, 7.60 mL, 104.2 mmol) was slowly added dropwise to a methanolic solution of D-serine (10.0 g, 95.1 mmol) and the reaction mixture was stirred for 24 h at room temperature. After completion of the reaction, the solvent was removed by rotary evaporator to give a white solid. The solid was washed with hexane (3 x 25 mL) to give D-serine methyl ester hydrochloride (14.4 g, 97% yield) as a colorless solid. The product was characterized by 1H NMR (300 MHz, D?O) with the following chemical shifts: δ 4.32-4.28 (1H, m, CH), 4.12 (1H, dd, J = 4.2, 12.5 Hz, CHaHbOH), 4.02 (1H, dd, J = 3.4, 12.5 Hz, CHaHbOH), 3.88 (3H, s, CH?) .
Yield:5874-57-7 91%
Reaction Conditions:
in methanol for 15.5 h;Reflux;
Steps:
1 Preparation of D-Serine Methyl Ester Hydrochloride
Example-1
Preparation of D-Serine Methyl Ester Hydrochloride
D-serine (100 g, 0.9515 mole) was suspended in 600 ml methanol at room temperature.
Acetyl chloride (224.0 g, 2.8545 mole) was added drop wise at -5° C. to 0° C. and stirred for 30 minutes.
The reaction mixture was refluxed for 15 hours.
Evaporation of the reaction mixture under reduced pressure followed by crystallization of the resulting residue from methanol and methyl tert-butyl ether, resulted in colorless solid of D-serine methyl ester hydrochloride (134.7 g, Yield: 91%, HPLC: 99.6%) M.R: 165-167° C. (Lit.: 163-165° C., Tetrahedron Letters, 2012, 53, 1668-1670,)[α]D20=3.7 (C=4 in EtOH); IR(KBr): 3361, 2921, 2660, 2732, 2550, 2488, 2134, 2079, 1922, 1747, 1592, 1505, 1471, 1444, 1431, 1382, 1343, 1297, 1258, 1187, 1158, 1128, 1094, 1038, 969, 900, 793, 844, 580, 469 Cm-1; H1NMR: (300 MHz, DMSO), δ3.745 (s, 3H), 3.82 (s, 2H), 4.11 (s, 1H), 5.63 (s, 1H), 8.58 (s, 2H); 13CNMR: (300 MHz, DMSO), δ 52.66, 54.37, 59.38, 161.39.
References:
Divi's Laboratories Limited;Divi, Satchandra Kiran;Rao, Mysore Aswatha Narayana;Nowshuddin, Shaik US9447024, 2016, B1 Location in patent:Page/Page column 11

67-56-1
790 suppliers
$7.29/5ml-f

312-84-5
628 suppliers
$5.00/1g

75-36-5
589 suppliers
$17.92/100G

5874-57-7
314 suppliers
$5.00/5g